Yuhan Corporation’s self-developed lung cancer treatment was approved for marketing in Korea. It is a Korean new drug (No. 31) that was released three years after K-Cap tablet (Gastroesophageal reflux disease treatment) released by HK Innoen in 2018. It is the treatment that received attention from global pharmaceutical company Janssen Biotech three years ago for technology exports of 1.4 trillion won.
As the lung cancer treatment market is rapidly growing and the treatment effect of new drugs is known to be excellent, there are also observations that it could become the nation’s first’global blockbuster new drug’ with annual sales exceeding 1 trillion won.
Expectation of Korea’s first’global blockbuster’
The Ministry of Food and Drug Safety announced on the 18th that it had approved Yuhan Corporation’s non-small cell lung cancer treatment, Rekrajajeong (ingredient name: Razertinib), to market. Recraza has not yet completed the phase 3 clinical trial, but the Ministry of Food and Drug Safety gave a conditional marketing permission for use in the medical field, considering the good treatment effect.
Non-small cell carcinoma, which is named as’the size of cancer cells is not small’, accounts for 80-85% of all lung cancer patients, which are about 30,000. Of these, 30-40% of them have mutations in T790M, an epithelial growth factor receptor (EGFR). Currently, these patients are given 1st and 2nd generation targeted therapies, but more than half of them soon become resistant to the drug and their condition worsens.
Rekraza is a third-generation targeted therapy for these patients. It has been approved for use in patients with T790M mutations who have developed resistance to the first and second generation EGFR target therapy. The company explained that it showed excellent efficacy in lung cancer patients that have metastasized to the brain.
Ahn Myung-ju, a professor of hematology and oncology at Samsung Medical Center, said, “Recraza has been recognized for its effectiveness and safety by being published in the world-recognized lancet oncology journal.
Yuhan Corporation plans to grow Rekraza into the first global blockbuster drug in Korea (over 1 trillion won in annual sales). This is because the non-small cell lung cancer treatment market is expected to grow rapidly from $19.2 billion in 2019 to $32.9 billion in 2029, and AstraZeneca’s Tagriso, which targets patients with Rekrazawa, has already raised annual sales to 1 trillion won.
An official from Yuhan said, “If you take advantage of Janssen’s global network, we will be able to raise Rekraza to a level that aligns with Tagriso.” This is more than 50% more than the expected annual sales of Rekraza (about 6247 billion won) by Global Data, a market research firm.
‘The 2nd Rekraza’ is waiting in line
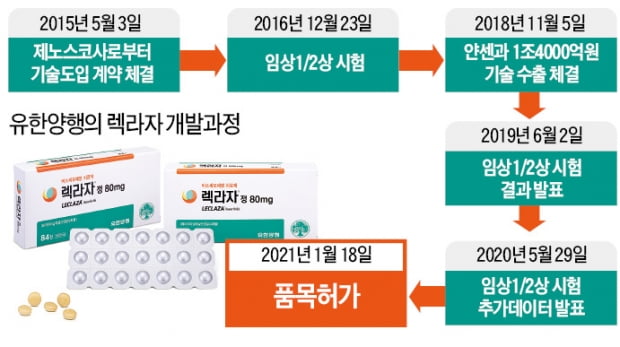
The process of developing Rekraza is quite different from the existing domestic new drugs. A pharmaceutical company alone found new drug candidates and then solved the problem through collaboration with domestic drug developers and global pharmaceutical companies, breaking away from the conventional method of developing them directly.
It started in 2015. Genosco, a subsidiary of Oscotech, a Korean drug developer, took over the right to develop a candidate for Lexaza, which was in the preclinical stage. For the next three years, Yuhan Corporation refined candidate substances into drugs through substance optimization, preclinical, and clinical processes, and then signed a technology transfer contract with Janssen in 2018. By joining hands with Janssen, the candidate substance gradually took shape as a global new drug.
An official from Yuhan Corporation said, “Typically, it takes more than 10 years and development costs in units of trillions to develop a new drug.” “The’open innovation’ method that develops new drugs through collaboration with other pharmaceutical companies We were able to save a lot of time and money.”
Yuhan Corp. plans to expand the collaboration method and release the second and third lekraza sequentially. Representative examples include a non-alcoholic steatohepatitis treatment (collaboration with Genexine, a new drug developer in Korea) and an immune anticancer drug candidate (collaboration with a domestic antibody drug venture company AbClon), which received KRW 1.5.2 trillion and exported technology to Boehringer Ingelheim in 2019. In this way, Yuhan Corporation has exported 5 new drugs to foreign countries, reaching 4 trillion won. An official from Yuhan Corporation said, “We have secured 22 of the 30 pipelines we currently have through open innovation.” “We will leap forward to become a’new drug brand’ by steadily increasing R&D investments.”
Reporter Oh Sang-heon [email protected]