 |
| On the morning of the 10th, a medical staff in charge of vaccination is waiting at the Daegu 1 novel coronavirus infection (Corona 19) vaccination center located at Keimyung University Daegu Dongsan Hospital in Jung-gu, Daegu. 2021.2.10/News1 © News1 Reporter Jeong Sik |
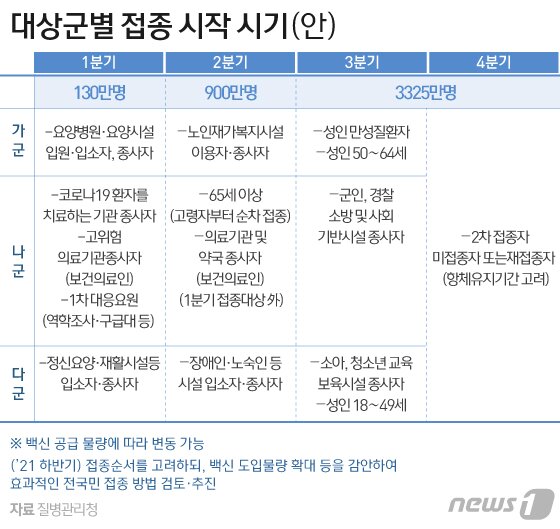
AstraZeneca’s new coronavirus infection (Corona 19) vaccine will begin its first vaccination in Korea around the 25th to 26th. Accordingly, attention is being paid to whether the Korea Centers for Disease Control and Prevention adjusts the vaccination plan for those over 65. The 65-year-old vaccination of the AstraZeneca vaccine is in a situation that differs from country to country.
In the domestic medical field, there are mixed opinions that the vaccination should be delayed until additional clinical data to be released in two months are confirmed, with the majority of opinions that elderly people 65 years of age or older should also perform vaccination as scheduled to form group immunity.
According to industry sources on the 11th, as the Ministry of Food and Drug Safety approved the domestic product license for AstraZeneca’s’Corona 19′ vaccine at the final inspection committee on the 10th, detailed vaccination plans such as the 65-year-old vaccination will be provided by the Korea Centers for Disease Control and Prevention Vaccination Specialized Committee. Placed in the hands of. The Agency for Disease Control and Prevention plans to receive expert advice in the near future and open a committee to determine the details of the vaccination plan for those over 65.
The Ministry of Food and Drug Safety approved the administration of the AstraZeneca vaccine to all ages over 18 years of age, and added a condition to submit additional clinical results for the elderly in the United States by April.
In addition, we added a cautionary caution that people over 65 should be vaccinated carefully. In the case of elderly people, the number of clinical participants is insufficient, and statistical analysis for that age is insufficient.
In this regard, Kang-Rip Kim, head of the Food and Drug Administration, said, “There is no problem in terms of safety and immune response in the case of elderly people 65 years of age or older (after vaccine administration), but additional data are needed, so that doctors can fully determine the benefits of vaccination according to the condition of the subject. I did,” he explained.
He also said, “At least in the content of the license as a prerequisite, we have not found a reason to limit the license for vaccination even for the elderly over 65 years of age.” Reasonable guidelines will be presented.”
Among experts, the opinion that vaccination for the elderly is acceptable is dominant. Choi Won-seok, a professor of infectious medicine at Korea University Ansan Hospital, said, “The data collected until December of last year are not statistically significant, but the overall effect shows a tendency that is not significantly different between the general adult and the elderly.”
Professor Nam Jae-hwan of the Department of Biomedical Science at Catholic University said, “The five vaccines we intend to introduce in Korea show an antibody production rate of at least 90% to almost 100%.” I would recommend it.”
On the contrary, there were also opinions that the vaccination plan of some preferential vaccination subjects should be changed. The vaccination for workers and residents of nursing hospitals and nursing facilities, which is the priority target of the AstraZeneca vaccine starting on the 26th, includes the elderly, and most of them suffer from underlying diseases, so the vaccination should be delayed.
An expert who requested anonymity said, “The urgent problem is that Corona 19 in the community is causing death by entering nursing hospitals and facilities,” and “It is not limited to cases with underlying diseases, but in the absence of relevant grounds, It is also necessary to consider delaying vaccination for the elderly with poor health.”
There is also a lot of prudence. Lee Hyuk, insurance director of the Korea Open Internal Medicine Association (Chief of JoongAng St. Mary’s Hospital), said, “Once we have to watch a little more, we will have to look at the position,” he said. said.
The Ministry of Food and Drug Safety has received the interim results of the AstraZeneca clinical trial, which is being conducted on 30,000 people (about 7500 elderly people) in the United States, by the end of April this year, and plans to delete the cautionary precautions for the elderly if there is no abnormality in safety.
According to the current vaccination implementation plan schedule, users of home-based welfare facilities for the elderly and those over 65 will start vaccination in the second quarter. As the KFDA decided to receive the data by April, the age may be free from controversy over careful administration precautions.
In this regard, Jung Eun-kyung, head of the Korea Centers for Disease Control and Prevention, said in the last briefing, “It is not that it is not effective for people over 65 years old, but that there is not enough data to judge the effect, so you have to make a careful decision.” I will decide whether to do it.”
 |
| © News1 Designer Eunhyun Lee |
