Sending time2021-02-28 13:17
comment
[앵커]
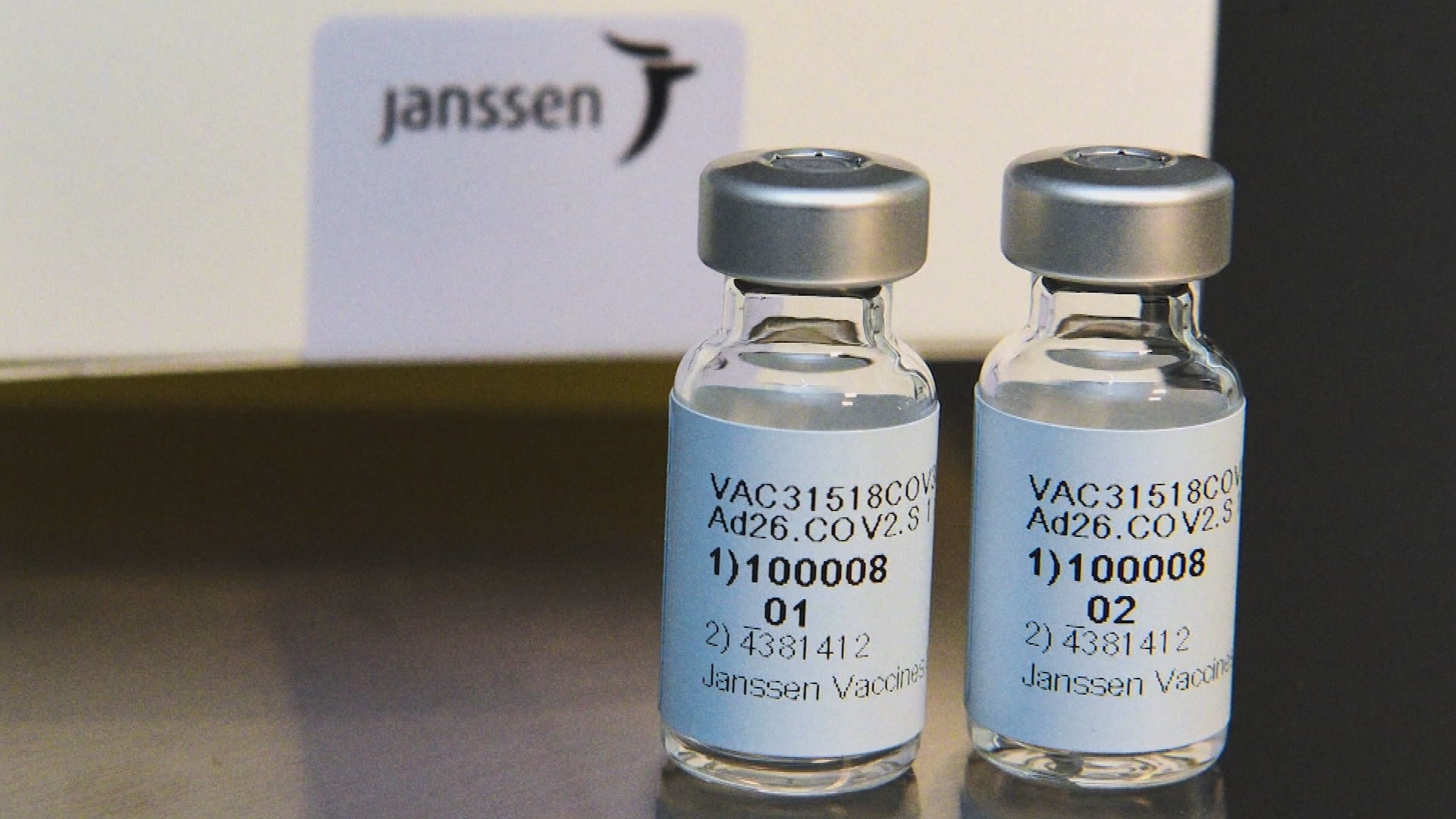
The U.S. Food and Drug Administration FDA has approved the emergency use of Johnson & Johnson’s COVID-19 vaccine.
With this, the United States has secured the third COVID-19 vaccine after Pfizer and Modena’s vaccine.
This is Park Hye-jun PD.
[기자]
The U.S. Food and Drug Administration FDA has approved the emergency use of Johnson & Johnson’s COVID-19 vaccine.
Previously, the Vaccine and Biological Drugs Advisory Committee, an advisory body, recommended the FDA for urgent use of this vaccine so that it can be given to adult Americans over the age of 18.
It is an analysis that the FDA’s approval for emergency use has passed an important gateway for commercialization because it is regarded as an expert certification procedure for the safety and efficacy of the vaccine.
With this, the United States has secured the third COVID-19 vaccine after Pfizer and Modena’s vaccine.
<프랜시스 콜린스 / 미 국립보건원 원장> “That’s really good news. This means that more people have a chance to get immunized against COVID-19 as soon as possible.”
Johnson & Johnson’s vaccine, unlike Pfizer Moder or vaccine, which requires two vaccinations, provides sufficient immunity with only one vaccination and can be stored in a general refrigerator.
In clinical trials conducted in the U.S. and South Africa, overall, it was found to show 66% efficacy in preventing mild and severe symptoms, but no one died of Corona 19 after getting this vaccine.
However, it was found that it did not affect the efficacy of Pfizer, Moder, or vaccine.
In response, Johnson & Johnson explained that it seems that the time when the clinical trial of its vaccine was conducted was after the emergence of the mutant virus.
This is Hyejun Park on Yonhap News TV.
Yonhap News TV article inquiries and reports: katok/line jebo23
Unauthorized reproduction-prohibition of redistribution>
2021/02/28 13:17 sent