Ministry of Food and Drug Safety approved for phase 1 and 2 clinical trials
 viewer
viewer
Ubiologics (206650)A clinical trial of a novel coronavirus infection (Corona 19) vaccine developed by
The Ministry of Food and Drug Safety announced on the 21st that it has approved the clinical trial plan of UBiologics’ Corona 19 vaccine’Yukobac-19′ on the 20th. As a result, 7 vaccines and 15 treatments are currently being developed with approval for a clinical trial plan related to Corona 19 in Korea.
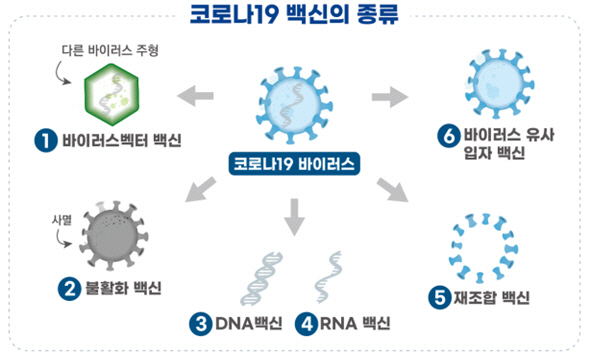
This clinical trial is to evaluate the safety and immunogenicity of Yukobac-19 in healthy adults. Phase 1 is followed by phase 2 in sequence. Yukobac-19 is a’recombinant vaccine’ made using genetic recombination technology of the’surface antigen protein’ of the Corona 19 virus.The surface antigen protein of the vaccine stimulates immune cells to form neutralizing antibodies to induce an immune response. When the virus invades, the antibody removes the Corona 19 virus.
Yukobaek-19 used liposomes as an adjuvant. Immunity enhancers are ingredients for enhancing the immune response of the vaccine and its clinical efficacy. As a vaccine, surface antigen proteins are expressed on the surface of liposomes to induce an immune response. Overseas, the US NovaVax (Phase 3) and others are conducting clinical trials of the COVID-19 vaccine using genetic recombination technology.
The Ministry of Food and Drug Safety announced that it will promptly deliver information on the status of clinical trials of the developed product, taking into account the high public interest in the development of COVID-19 treatment and vaccine.
/ Reporter Woo Young-tak [email protected]
< 저작권자 ⓒ 서울경제, 무단 전재 및 재배포 금지 >