GC Green Cross plans to apply for permission in April after finishing data cleanup within the first quarter
(Seoul = Yonhap News) Reporter Jandi Kim = Chong Kun Dang[185750]’Nappaveltanju’ (ingredient name Napamostat mesylate), a new coronavirus infection (Corona 19) treatment, is getting a lot of attention as to who will take the place of the’domestic No. 2’treatment, drinking from a conditional license.
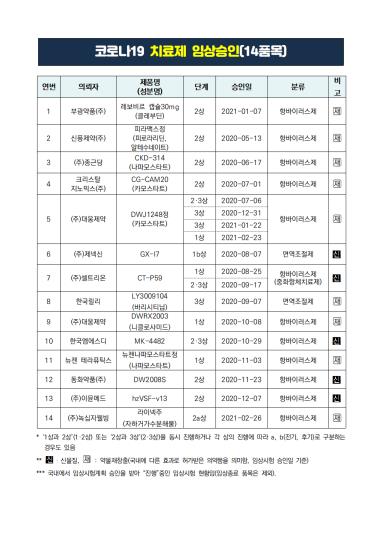
According to the Ministry of Food and Drug Safety on the 18th, there are two types of Corona 19 treatments approved in Korea: Gilead Science Korea’s Beculuriju (ingredient name remdesivir) and Celltrion’s Rekironaju (ingredient name regdanvimab).
Among them, Rekironaju, which was approved on the 5th of last month, is the’domestic No. 1’Corona 19 treatment developed in Korea.
Chong Kun Dang, GC Green Cross, and Daewoong Pharmaceutical have been mentioned as domestic pharmaceutical companies that have taken the position of the second domestic drug since Celltrion was honored for the first time.
Since then, Chong Kun Dang applied for an item permission from the Ministry of Food and Drug Safety on the 8th of this month, and there were many evaluations that it was the most advanced.
Although Chong Kun Dang’s approval failed, expectations for a domestic COVID-19 treatment have declined, but GC Green Cross is in the midst of working on a final application for a corona 19 blood system drug license.
GC Green Cross completed the drug administration of the corona19 blood system drug’GC5131A’ for the clinical phase 2 test subjects on December 31 of last year, and has analyzed and summarized related data so far.
An official from GC Green Cross said, “The data is currently being organized,” and “after completing the analysis within the first quarter, we plan to apply for permission to the Ministry of Food and Drug Safety in April.”
The fact that GC Green Cross’s corona19 blood treatment system is being used by patients in medical fields through approval for therapeutic use by the Ministry of Food and Drug Safety is raising expectations before approval. From October last year to March 9 this year, a total of 41 treatment purposes were approved.
The Ministry of Food and Drug Safety is operating a treatment-purpose use approval system that allows users to use drugs for clinical trials that are not licensed for the treatment of severe, life-threatening patients or other treatment means.
Daewoong Pharmaceutical is also conducting phase 2/3 clinical trials to develop Hoystar tablet (ingredient name chamostatmesylate) as a treatment for Corona 19.
The specific permit application plan was not disclosed. Daewoong Pharmaceutical is also conducting phase 3 in parallel to confirm the effects of Hoystar tablets on the prevention of Corona 19.
In addition, among domestic pharmaceutical companies, Bukwang Pharmaceutical[003000], Shinpoong Pharmaceutical[019170], Crystal Genomics[083790], Dong Wha Pharm[000020], Immunmed, Green Cross Wellness[234690] Et al. are conducting a phase 2 clinical trial to develop a treatment for COVID-19.
![[식품의약품안전처 제공. 재판매 및 DB 금지]](https://i0.wp.com/img5.yna.co.kr/etc/inner/KR/2021/03/17/AKR20210317183900017_01_i_P4.jpg?w=560&ssl=1)
[식품의약품안전처 제공. 재판매 및 DB 금지]
Unauthorized reproduction-redistribution prohibited>
2021/03/18 07:00 sent