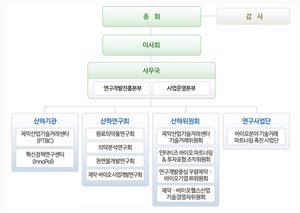
[이코노믹리뷰=황진중 기자] The Korea New Drug Development Research Association announced on the 24th that as of the 1st, it divided the secretariat organization into the R&D Promotion Headquarters and the Project Operation Headquarters and reorganized into a four-team system. An official of the New Drug Development Research Association said, “As a representative organization in charge of research and development of innovative pharmaceutical and bio new drugs in the biohealth industry, it is to prepare for the post-corona era, to support global open innovation of member companies, to promote the development of new pharmaceuticals and bio drugs, and to support business expansion.” Explained.
The R&D Promotion Headquarters (R&D Policy Planning Team, Business Development Team) is in charge of policy for global growth of the pharmaceutical and biohealth industry, domestic and international industry-academia-research cooperation, open innovation, and industrial support. .
The Project Operation Division (Planning Budget Team, External Cooperation Team) is in charge of establishing and managing the union budget plan, public relations with the press, and overall cooperation with the government and related organizations, and Jae-cheon Yeo (Secretary General) will serve as the head of the headquarters and lead Jindu.
As a result, the New Drug Development Research Association has established an organization that fits the leadership in the development of biohealth pharmaceuticals and bio new drugs in the nation’s big three industries.
Meanwhile, currently affiliated with the Korea New Drug Development Research Association, there are the Pharmaceutical Industry Technology Transaction Center (PTBC) and the Innovation Policy Research Center (InnoPol). Subsidiary research circuits include the Drug Raw Materials Research Group, the Pharmaceutical Analysis Research Group, the Natural Products Development Research Group, and the Pharmaceutical and Bio Business Development Research Group (K-BD Group).
Subsidiary committees include the Pharmaceutical Industry Technology Exchange Center Technology Transaction Committee, Interbiz Bio Partnering & Investment Forum Organizing Committee, R&D-centered superior pharmaceutical and bio company IR committee, pharmaceutical and biohealth industry technology manager committee, and subsidiary research divisions in the bio field. It is actively operating a technology transaction partnering promotion project group.