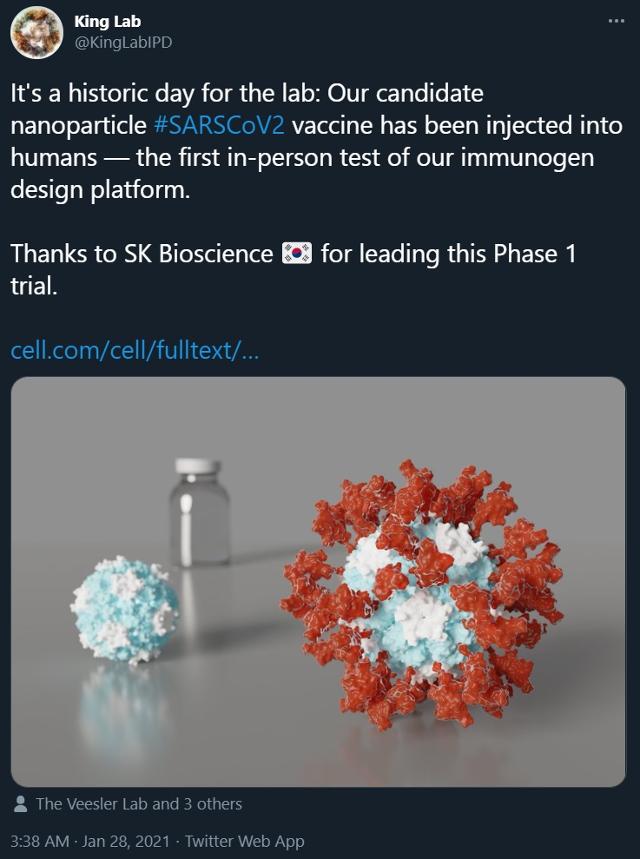
The team of Professor Nail King of Washington University in the U.S., who is jointly developing a COVID-19 vaccine with SK Bioscience, revealed on their Twitter account that they have started a phase 1 vaccine experiment. Neil King University of Washington professor team Twitter
Phase 1 clinical administration of’GBP510′, the second novel coronavirus infectious disease (Corona 19) vaccine candidate being developed by SK Bioscience, has begun.
According to the industry on the 31st, the team of Professor Nail King of Washington University in the United States, who is jointly developing the vaccine candidate, said, “Our Corona 19 vaccine candidate was administered to humans” through a Twitter Lab account on the 28th (local time). “It is the first time that our immunogen platform is tested in humans.”
GBP510 is a’recombinant vaccine’ that uses genetic recombination technology to make the surface antigen protein of the Corona 19 virus. When the surface antigen protein of the vaccine stimulates immune cells, neutralizing antibodies are formed, which induces an immune response. When the Corona 19 virus invades, antibodies will remove the virus.
GBP510 is a candidate for a COVID-19 vaccine that is being developed jointly with the University of Washington Antigen Design Research Institute by SK Bioscience in May of last year with a grant from the Gates Foundation. At the end of December last year, it was approved for phase 1 and 2 clinical trials by the Korean Food and Drug Administration. Prior to this, it is separate from SK Bioscience’s COVID-19 vaccine candidate’NBP2001′, which was approved for clinical trials at the end of last November.
Ryu Jong-eun reporter [email protected]
News directly edited by the Hankook Ilbo can also be viewed on Naver.