 |
| A new coronavirus infection (Corona 19) vaccine was administered to medical staff by itself, and medical staff at Seoul National University Hospital in Jongno-gu, Seoul are looking for adverse reactions after vaccination./News1 © News1 Reporter Park Ji-hye |
On the 5th, the Ministry of Food and Drug Safety finally approved the new coronavirus infection (Corona 19) vaccine’Cominati’ from Pfizer, a US pharmaceutical company. In the same way as the Central Pharmacist Review Committee (hereinafter referred to as the Central Pharmacopoeia), which is the legal advisory body of the Ministry of Food and Drug Safety, it has been decided that the drug can be administered by persons aged 16 years or older.
Most of the 16-year-olds in Korea are first graders in high school. It is a way for high school 1st to 3rd graders who have passed their birthday to proceed with the Corona 19 vaccination. Until now, there has been no COVID-19 vaccine in Korea that children and adolescents under the age of 18 can administer. However, the situation changed as the age of Pfizer vaccine administration was set at 16.
However, there are more procedures that high school students must go through to actually proceed with vaccination. First of all, the Vaccination Committee of the Korea Centers for Disease Control and Prevention, which oversees the vaccine policy, should decide on vaccination for middle and high school students among children and adolescents.
◇ Three times verified by the Ministry of Food and Drug Safety “Permission for over 16 years old”… The first vaccine available for use by minors
Kang-rip Kim, the head of the Ministry of Food and Drug Safety, said at a briefing on the 5th, “The final inspection committee approved the product under the condition of submitting the final results of clinical trials for Pfizer vaccine’Cominatiju’.” He added, “In the same way as the results of the previous two consultations, we judged that not only the efficacy and effect, but also permission for those aged 16 years or older is valid.”
“The final inspection committee judges that the safety of Pfizer’s vaccine is good,” he said. “Abnormal cases are also predicted by vaccine administration,” he said. Abnormal cases seen in vaccine clinical trials include pain, fever, fatigue, chills, headache, and muscle pain in the body part that received the injection. Most are mild, and symptoms disappeared within a few days after vaccination.
Of all 43,448 clinically enrolled subjects, four’significant adverse drug reactions’ that could not be ruled out related to vaccine administration were confirmed. Among them, lymphadenopathy and ventricular arrhythmia were recovered. Shoulder wounds, request and bilateral lower limb pain are recovering. The effectiveness of the Pfizer vaccine was judged to be sufficient with a prevention effect rate of about 95%.
Currently, the Ministry of Food and Drug Safety is operating a triple verification system for the Corona 19 vaccine. The safety and effectiveness of vaccines are verified through three steps: the verification advisory group → central pharmaceutical review → final inspection committee. The final inspection committee is the last stage of consultation and verification by the Ministry of Food and Drug Safety, following the verification advisory group held on February 22 and the Central Pharmaceutical Affairs hearing held on the 25th.
In Korea, the Pfizer vaccine is being administered by medical staff at the Corona 19 treatment hospital. As of 0 o’clock on the 5th, 3,909 people received the Pfizer vaccine. No serious adverse reactions such as death cases or anaphylactic shock have been reported after vaccination. However, compared to overseas cases, there is a high possibility that adverse reactions to Pfizer vaccines will also be reported at a rate of 0.1 to 0.2%.
‘Cominatiju’ is a gene mRNA vaccine jointly developed by Pfizer and Bioentec, Germany. Since this vaccine is composed of mRNA and lipid nanoparticles (LNP), it is not a chemically tight bond, so the final inspection point was to include’smooth inversion’ and’not shaking’ in the precautions for use.
The efficacy and effectiveness of this drug prevents Corona 19 in people 16 years of age or older. For the usage and dosage, after diluting the stock solution, inoculate 0.3 milliliter (mL) once, and then inoculate an additional 3 weeks later. Storage conditions are 6 months at -60 to 90 degrees Celsius. However, the U.S. Food and Drug Administration (FDA) announced in a statement on the 25th (local time) last month that it allowed the method to store and deliver the frozen Pfizer vaccine stock solution from “between -25 degrees and -15 degrees Celsius” for up to two weeks. .
There is a high possibility that the same decision will be made in Korea as well. “Clinical data such as those submitted by Pfizer to the FDA have not yet been submitted,” said Kim Kang-rip, head of the Food and Drug Administration. “We will discuss and decide when related data are submitted.”
 |
| On the morning of the morning of the 3rd, the first day of the Pfizer vaccination by region, medical staff are waiting to receive the Pfizer vaccine at the Corona 19 Vaccination Center in the central region of Cheonan, Chungcheongnam-do. (Photography Foundation)/News 1 |
◇ The burden of reporting side effects for minors is high… Pay attention to parents, vaccination consent rate, etc.
High school students’ COVID-19 vaccination requires a more thorough preparation against the risk of side effects such as anaphylactic shock as the target of the vaccine is minor. In the first half of last year, after the report of the death of a minor suspected of being infected with Corona 19 was received, the whole country was shaken by the precedent, and attention is focused on what decision the Korea National Disease Service Vaccination Committee will make.
The most worrisome side effects are severe adverse reactions such as anaphylactic shock. Anaphylaxis shock is an allergic hypersensitivity reaction, when an allergic reaction occurs to certain substances used in vaccine production. An anaphylaxis reaction is also caused by a bee sting during a bee sting and a shock or a sudden airway obstruction after eating food and breathing difficulties.
This is different from the cytokine storm, which is an excessive immune response. According to the Centers for Disease Control and Prevention (CDC), the probability of developing anaphylaxis after all vaccinations, as well as flu vaccines, is 1.31 cases per 1 million vaccinations.
Parental consent is expected to be required when vaccinating Pfizer vaccinations for high school students who are minors. You should also go through a procedure to examine the consent rate for vaccinations for parents and students. The consent rate for the COVID-19 vaccine vaccination for large domestic hospitals such as Seoul National University Hospital recorded 88%.
Last month, when the quarantine authorities surveyed workers under the age of 65, nursing hospitals under the age of 65, and workers in treatment hospitals for COVID-19 patients, 93.8% agreed to vaccination. 93.6% of the 30,8930 people who received the AstraZeneca (AZ) vaccine and 94.6% of the 5,829 Pfizer vaccine targets agreed to the vaccination.
However, the results of the survey on minors are likely to be lower than this. Experts contend that unnecessary concerns about vaccines should be reduced. “Vaccines have great advantages and are the first step in our society, so we have to have trust,” said Kim Yeon-soo, director of Seoul National University Hospital, who vaccinated the AstraZeneca vaccine on the 4th. Believe it and be right.”
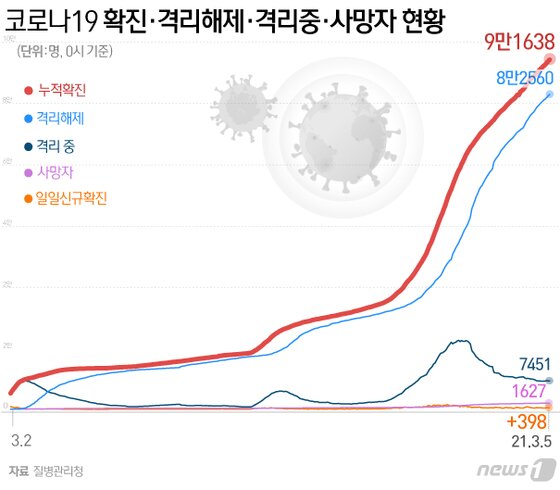 |
| © News1 Designer Eun-Hyun Lee |
