 |
| On the afternoon of the 15th, patients are coming out of the hospital after seeing outpatient treatment in front of a hospital in Gwangju. According to the city of Gwangju, two medical staff at the hospital were confirmed to have a novel coronavirus infection (Corona 19) the day before. 201.2.15/News1 © News1 Reporter Daum Jung |
The government has decided to temporarily postpone vaccination of the vaccine against AstraZeneca’s novel coronavirus infection (Corona 19) over the age of 65. Due to the controversy over efficacy due to lack of clinical data, it was decided to first resolve the public anxiety about vaccination.
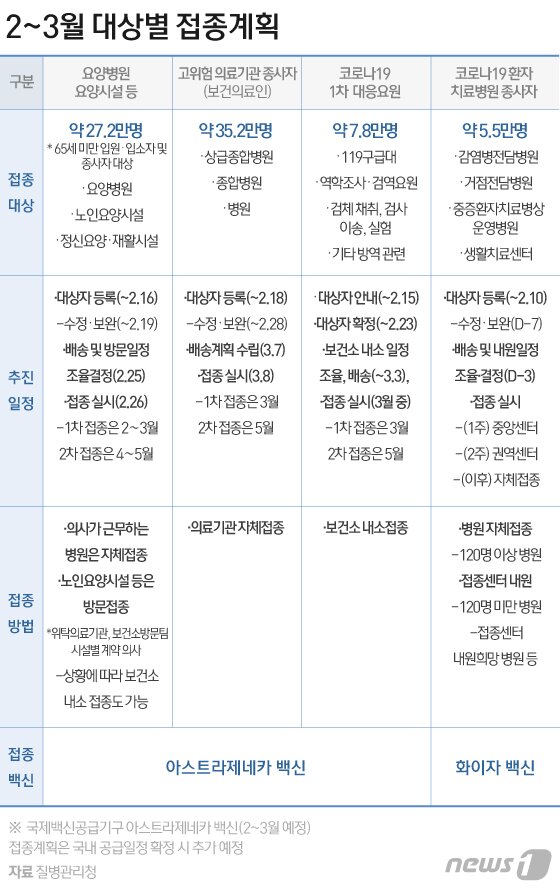
Accordingly, the domestic Corona 19 vaccination in February-March this year is first applied to the hospitalized, residents, and workers under the age of 65 in group facilities for the elderly, such as nursing hospitals and nursing facilities, using the AstraZeneca vaccine.
The COVID-19 vaccination response team plans to confirm additional clinical information on the effectiveness of the vaccine at the end of March for those 65 years of age or older, and then determine the vaccination plan through deliberation by the Vaccination Specialist Committee.
The following is a question and answer from Jung Eun-kyung, head of the Corona 19 Vaccination Response Promotion Team (Director of the Disease Control and Prevention) and reporters.
-If you are 65 years of age or older, why are you procrastinating?
▶ AstraZeneca vaccine is a vaccine that has been conditionally licensed or approved for emergency use by verifying its safety and effectiveness in 50 countries including Korea as well as the European Medicines Agency. Safety was secured even in the 65-year-old or older, and the immune response to form antibodies was also confirmed. However, since the number of clinical trial participants over 65 years of age was small, the number of infected people in the vaccine group and control group was very small, so there was a limit to derive statistical significance for effectiveness. In consideration of these points, additional efficacy information will be confirmed, and an inoculation implementation plan will be prepared for those over 65 years of age.
-Is there any impact on the original goal of 70% vaccination by September?
▶ There are two major variables that affect the achievement of the 70% vaccination target by September. One is the timing of domestic vaccine supply, which can affect the supply uncertainty depending on the world vaccine production volume and demand. The second is the vaccine effect due to the mutant virus. We will try to increase the timing of vaccine supply, considering how much the mutant virus will be in Korea as a variable.
-What is the deadline for submitting additional data for vaccination over the age of 65?
▶The clinical trial ends around the end of March. Since it takes a little time to analyze and derive the results, it is necessary to further confirm the timing of the accurate submission. I know that the Ministry of Food and Drug Safety requested that the submission be submitted by April. Even before that, if other scientific evidences can be continuously collected and the effects can be judged, the vaccination plan can be adjusted at any time after deliberation by the Vaccination Committee.
-Who is eligible for the first domestic vaccination?
▶ As for those who are eligible for No. 1 vaccination, AstraZeneca vaccination will be sequentially expanded from February 26 to nursing hospitals and nursing facilities, so it is expected that nursing hospital workers will be eligible for No. 1 vaccination. Since the vaccination plan is still being confirmed by local governments, we will inform you when a detailed vaccination schedule is determined.
-Who is eligible for vaccination in nursing hospitals and nursing facilities under the age of 65 who give priority vaccination?
▶ In the case of nursing hospitals, 1,720 are eligible. Among them, the number of vaccination targets under the age of 65 is estimated to be about 30,000 hospitalized patients and 137,000 workers. There are 4153 nursing facilities subject to vaccination, and it is estimated that about 11,000 people under the age of 65 and 90,000 people are employed.
-Is there a possibility that vaccinations over 65 will be delayed?
▶From the conclusion, it does not have a significant effect on the formation of group immunity. I don’t think that the 370,000 people who are scheduled to be vaccinated in the second quarter will be given a lower priority. This is because I believe that the vaccination should be started at least in the second quarter. The government will consider these areas and consult with experts to prepare an inoculation plan. I will manage it so that it does not go up to the third priority.
-There is also a concern about the occurrence of vaccination “overflow” during the second quarter
▶There is an opinion that if the inoculation passes in the second quarter, it may be burdensome to administer vaccinations, but it is expected that it will be possible to prepare systematically. Influenza vaccination, for example, starts in September and approximately 15 million people are vaccinated in about two months in October and November. The effective variable is rather the vaccine supply.
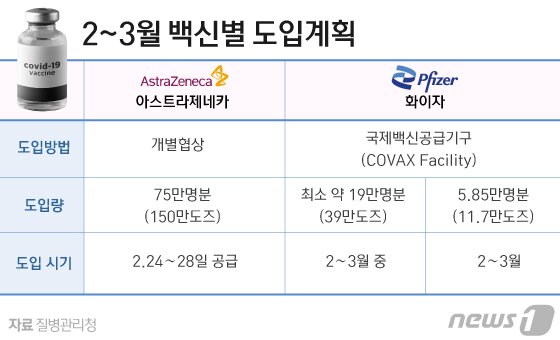
-Has there been a schedule for the vaccine supply for COVAX yet?
▶We are continuing to discuss with Kobax. About 2596,000 doses of AstraZeneca vaccine and 117,000 doses of Pfizer vaccine have been confirmed. Since administrative procedures are carried out simultaneously with so many countries, there are delays in procedures such as supply timing.
-What is the progress of purchasing additional vaccines?
▶ The introduction of 40 million doses of vaccine with NovaVax is being discussed continuously, and it is almost in the final stage. In addition, we are continuing to adjust the timing of securing or supplying additional quantities for vaccines that have already been contracted. Since the issue of mutations has been continuously raised in recent years, it means that it will open the possibility of various vaccine platforms and types.
 |
| © News1 Designer Ilhwan Kim |
 |
| © News1 Designer Eunhyun Lee |
![[Q&A] When to get vaccination over 65 years old… No. 1 in the second quarter at the latest [Q&A] When to get vaccination over 65 years old… No. 1 in the second quarter at the latest](https://image.news1.kr/system/photos/2021/2/15/4621327/article.jpg)