Reporter Seongmin Kim, Bio Spectator
Spectrum completes a meeting with the US FDA based on the clinical results of Cohort 2. Cohort 3 and Cohort 5 additionally disclosed clinical results
Spectrum Pharmaceuticals is pushing ahead with an application for US approval next year for the anticancer drug’poziotinib’ developed by Hanmi Pharmaceuticals.
Spectrum was presented on the 22nd (local time) with the US Food and Drug Administration (FDA) at a pre-NDA meeting based on the clinical results of Cohort 2 for HER2 Exon20 mutant-positive non-small cell lung cancer (NSCLC) patients with past treatment experience. They agreed to submit a new drug license (NDA) to Nip and announced that they will apply for a new drug license to the FDA next year.
Cohort 2 was a clinical trial that administered pogiotinib 16mg once a day (QD) to 90 patients with non-small cell lung cancer, and the objective response rate (ORR) was 27.8%. The median duration of response (mDOR) was 5.1 months, the follow-up period was 8.3 months, and the mPFS (median progression-free survival) was 5.5 months.
Joe Turgeon, President of Spectrum Spectrum, said, “The agreement with the FDA in the discussion for NDA application is an important milestone for Pogiotinib.” “The safety and effectiveness of Pogiotinib in areas with high unmet medical needs have a significant impact. I expect to be crazy.”
On the other hand, the spectrum revealed two additional data as a result of another study of Pogiotinib.
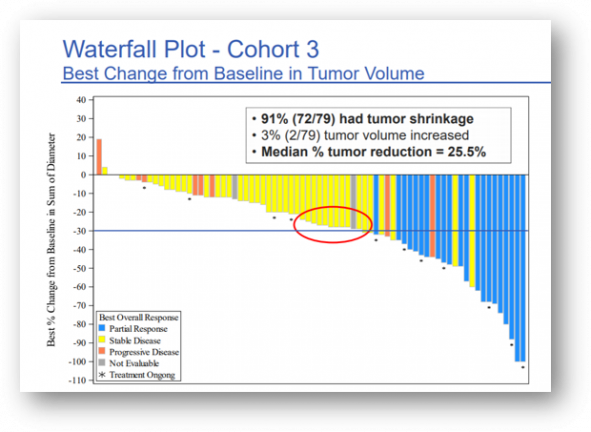
First, the results of Cohort 3 were announced, and this is a clinical trial evaluating the efficacy of Pogiotinib as a first-line treatment for EGFR Exon20 mutant-positive non-small cell lung cancer patients without treatment experience. Partial response (PR) was confirmed in 27.8% (22/79 patients) after administering a 16mg dose to 79 patients once a day.
The ORR according to the ITT (Treatment Intent Population Including Randomly Assigned Patients) analysis was 27.8%, and the total ORR range was 18.4 to 39.1% (95% confidence interval), but in some patients, it was based on a predefined statistical hypothesis that withdrawal occurred. As the expected minimum effective value (20%) of ORR was not reached, the first endpoint of cohort 3 was not satisfied.
However, DCR (disease control rate) was 86.1%, mDOR was 6.5 months, and PFS (progression free survival) was significantly improved 7.2 months, and the safety profile was similar to the side effects observed in other 2nd generation EGFR TKIs.

▲ Clinical results of Pogiotinib as a first-line treatment for EGFR Exon20 mutant-positive non-small cell lung cancer patients without cohort 3 treatment experience, spectrum presentation data
Next, the spectrum also revealed data from Cohort 5 that confirmed the safety of Pogiotinib according to the change in drug administration method. Cohort 5 was an extended study of non-small cell lung cancer patients with EGFR or HER2 Exon20 mutation, regardless of past treatment, and was administered twice a day (BID, 8 mg per dose). It was confirmed that the drug safety was improved through the changed dosage from once a day to twice. Compared to the existing once daily (QD, 16 mg per dose), the incidence of grade 3 or higher side effects such as rash, diarrhea, and stomatitis decreased by 32%. Also, the dose interruption decreased by about 38% compared to once a day (QD). No new types of adverse reactions (AEs) were observed in the twice daily dosing regimen.
Francois Lebel, Spectrum Chief Medical Officer (CMO), said, “Cohort 5 data administered with a new regimen twice a day significantly improves patient tolerability, and it is hypothesized that it reduces grade 3 or higher side effects by about a third. “I believe that improving the tolerance and reducing the discontinuation of taking the drug can improve the antitumor effect in various EGFR and HER2 clinical trials, thereby helping the overall clinical trial of Pogiotinib.”

▲Pogiotinib dose change cohort 5 safety results, spectrum presentation data