|
The Korea Pharmaceutical Bio Association announced on the 18th the results of the’Pharmaceutical Bio Industry ISO 37001 Certification Project Introduction Effect Analysis Study’ conducted by the Transparency Agency of Korea for about 6 months from August last year to January this year. Transparency Korea, which conducted the research, is the headquarters of Transparency International, an international non-governmental organization established in 1993 with the aim of overcoming international and national corruption.
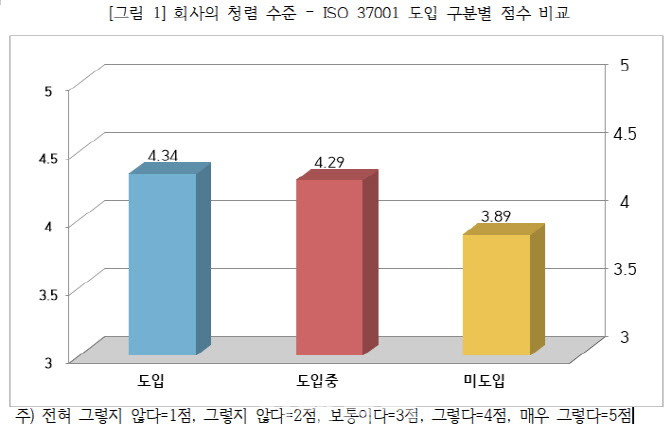
In this study, △Anti-corruption ethical culture (transparent handling of work, solicitation, etc.) △Anti-corruption system (corruption reporting system, etc.) △Integrity in internal and external work (personnel, money and gifts, etc.) ), etc., the level of integrity of companies that introduced ISO 37001 was 4.34 out of 5 out of 5, which was higher than that of companies that were adopting (4.29 points) and companies that did not (3.89).
In addition, when asked about the reason for the positive change in thinking about integrity and ethical behavior in the last 1-2 years, companies introducing ISO 37001 answered that’participation in the ISO 37001 program such as education and risk assessment’ had the greatest impact. On the other hand, companies that are adopting ISO 37001 pointed out that’the CEO’s anti-corruption and integrity will’, and non-introduced companies,’social anti-corruption atmosphere’, respectively, contributed to the change in employee ethics. Changes in attitudes toward anti-corruption of executives, executives, and fellow employees were also a factor in improving ethical awareness.
In order to increase objectivity, the Transparency Korea organization randomly selected survey subjects from a list of 4200 pharmaceutical company executives and employees based on the results of 1620 people.
Transparency Korea cited the interest and will of the CEO as the most important factors for the success of ethical management through its proposals. As with the introduction of the system, the organization of a dedicated organization and strengthening authority, active support of internal auditor activities, execution-evaluation-improvement, etc. It emphasized that all employees need to participate in the process, establish a channel for reporting corruption, and continue education and improvement.
Hee-mok Won, chairman of the Korea Pharmaceutical Bio Association, said, “ISO 37001 certification is a process for the company to establish an ethical management infrastructure.” “I will continue the ISO 37001 certification business this year and make efforts to establish ethical management of member companies by actively utilizing the research results. said.