[서울=뉴스핌] Reporter Wonjin Choi = US pharmaceutical company Pfizer and Bioentech have launched a clinical trial for the safety of their Corona 19 (COVID-19) vaccine for pregnant women.
 |
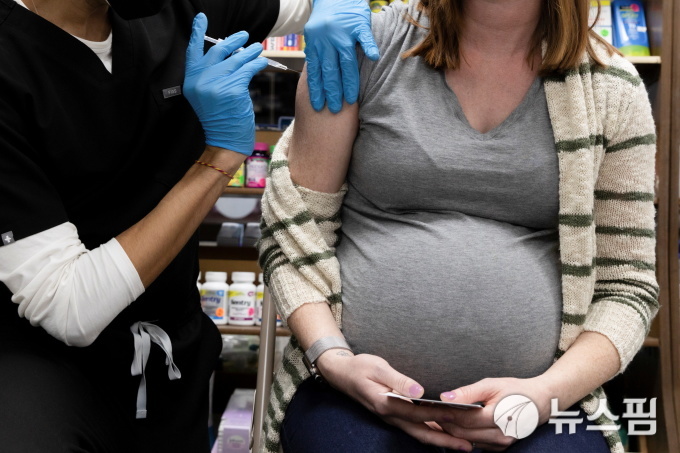
| Pregnant woman getting COVID-19 vaccine at a pharmacy in Schwenksville, Pennsylvania, USA. 2021.02.11 [사진=로이터 뉴스핌] |
According to Reuters on the 18th (local time), Pfizer’s William Gruber, vice president of vaccine clinical trials at the Pharmaceutical Research and Development Department, made the same statement.
The clinical trial is conducted in women over 18 years of age and 24 to 34 weeks of pregnancy. Participants are divided into vaccinated and non-vaccinated groups. The vaccinated group will receive two vaccines every 21 days.
This clinical trial is extensively studied in the United States, Canada, Argentina, Brazil, Chile, Mozambique, South Africa, the United Kingdom, and Spain.
Dr. Gruber said that the Corona 19 data so far show that the possibility of severe deterioration in pregnant women patients is relatively high, and hopes the vaccine will be effective for pregnant women as well.
Last week, the National Institutes of Health (NIH) ordered pharmaceutical companies to extend vaccine safety studies to pregnant and breastfeeding women.
Until now, clinical trials have not been conducted on pregnant women due to the safety problem of the vaccine. If the results of this test are successful, the possibility of large-scale vaccination of pregnant women is open.
The test further checks whether antibodies are formed in babies after childbirth of vaccinated pregnant women.
Pregnant women in the non-vaccinated group benefit from Pfizer vaccination after childbirth.