[서울=뉴스핌] Reporter Kim Joon-hee = RNA therapy platform company Olipass Co., Ltd., on the 8th, regarding the’unblinding’ of the Australian clinical trial phase 1b of the new non-narcotic analgesic drug OLP-1002, the pain evaluation value of the placebo group was unexpectedly decreased compared to the analgesic group. It was announced that special matters such as the occurrence occurred.
Olipass aims to evaluate the safety of OLP-1002, a new non-narcotic analgesic drug, targeting more than 30 patients with chronic arthritis pain.This’placebo-controlled double-blind’ clinical trial combines preparatory analgesia efficacy evaluation for exploratory purposes. Carried out. After administration of OLP-1002 and placebo 5 times over 2 weeks, it was designed to evaluate the change in pain over 6 weeks by VAS and WOMAC methods.
 |
| [로고=올리패스] |
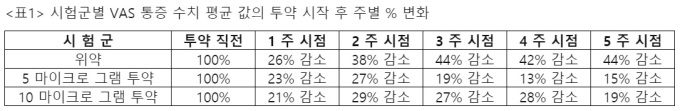
Olipass temporarily statistically processed pain data for each medication group based on the’unblinded’ data recently received from the CRO in charge of the clinical trial. This result <표 1>As shown, a significant reduction in pain levels was observed in the placebo group compared to the drug-treated group. This is a very unusual result considering the characteristics of chronic pain experienced by the target patient group.
Dr. Young-Rae Kim, vice president of clinical development for Olipass, a specialist in pain and rheumatology, said, “It will be clear only when the final results of this clinical trial are analyzed, but more than 70% of pain reduction was observed in 5 out of 10 people in the placebo group, whereas there was no pain reduction in 4 people in the placebo group. “There was no statistically significant difference in pain reduction between the and administration groups,” he said. “Considering that the changes in VAS and WOMAC pain levels for each patient were generally observed, the reliability of the evaluation method was high, so a detailed review of the patients in the placebo group. “I need it.”
Mental Olipath said, “In the Australian clinical trial, 8 patients who were close to painlessness were observed, so we had high expectations, but it is unfortunate that the pain reduction in the placebo group was observed abnormally large,” he said. “In the corona pandemic situation, we struggled with the patient who was willing to participate in the clinical trial. I am very grateful to the clinical staff,” he said.
As the safety was confirmed through this clinical trial, Olipass said that the European phase 2a trial for neurologically damaging pain patients and the phase 2a clinical trial for chronic arthritis pain patients will proceed within this year as scheduled. In addition, the company plans to thoroughly analyze the cause until the final result of this clinical trial is drawn, and make every effort to establish a clinical protocol in the future.
 |
| [사진=올리패스 제공] |