31st verification advisory group meeting… Results released on February 1st
Thorough verification of efficacy and stability for the elderly
 viewer
viewer
 viewer
viewer
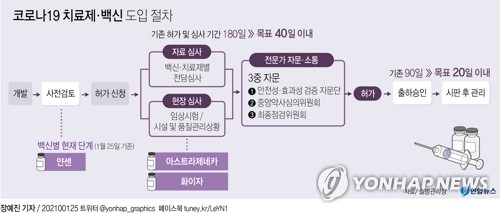
The Korean government’s approval screening process for AstraZeneca’s novel coronavirus infection (Corona 19) vaccine, which has been approved for use by the European Union (EU), is also in full swing. The authorities will hold the first expert meeting this weekend during a total of three expert advisory procedures and disclose the results on February 1.
According to the pharmaceutical industry on the 30th, the Ministry of Food and Drug Safety has a’Corona 19 Vaccine Safety and Effectiveness Verification Advisory Group’ (hereinafter referred to as the Verification Advisory Group) meeting on the afternoon of the 31st in which external experts participate in the clinical trial data of the AstraZeneca Corona 19 Vaccine (AZD1222). Open. The results will be released on February 1.
The Ministry of Food and Drug Safety goes through three external expert procedures for approval of the COVID-19 treatment and vaccine. It consists of the verification advisory group, the central pharmacist review committee (central drug review), and the final inspection committee. At the verification advisory meeting, clinical experts focused on infectious medicine will participate in the meeting to look into the safety and effectiveness of AstraZeneca Corona 19 vaccine, clinical significance, and appropriateness of target patients.
AstraZeneca, the first of the vaccines to be introduced in Korea, applied for an item license from the Ministry of Food and Drug Safety on January 4 this year. This product is a vaccine that is administered twice in total and can be transported under a separate distribution system because the storage conditions are 2-8℃. In addition, it is expected that rapid supply and demand will be possible because the consignment manufacturing company is produced in Korea with SK Bioscience. Korea has signed a vaccine purchase contract with AstraZeneca for 10 million people.
However, AstraZeneca recently raised a controversy in the German media when raising a question about the efficacy of the elderly over the age of 65. Accordingly, the Ministry of Food and Drug Safety is comparing and reviewing the vaccination group and the placebo group to see if there is any safety information that requires special attention from the elderly over 65 years old.
Although there have been some problems, the controversy seems to be calming down once the European Union (EU) executive committee approved the conditional sale of AstraZeneca’s COVID-19 vaccine on the 29th (local time). The European Medicines Agency (EMA) said that the AstraZeneca vaccine was found to be safe and effective in preventing COVID-19 in people over the age of 18, when combined with the results of clinical trials conducted in the UK, Brazil and South Africa.
/ Reporter Woo Young-tak [email protected]
< 저작권자 ⓒ 서울경제, 무단 전재 및 재배포 금지 >