According to the investment industry on the 22nd, Qrocell will issue a free increase for shareholders listed on the stockholders list as of the 31st. The par value is 500 won per share, and 9 new shares of the same stock are issued per share, and the number of new shares generated is 9.636165 shares (75,578,844 common stocks, 2,78361 preferred stocks). The investment industry believes that it will increase the number of shares in circulation in order to be listed in earnest. The company plans to apply for a technology evaluation in the first half of the year with the aim of listing this year.
Qrocell is the first domestic CAR-T treatment company established in 2016 by CEO Gunsu Kim, a new drug development expert who went through LG Life Sciences and Cha Biotech, Professor Chanhyuk Kim of KAIST, an authority on immune cells, and Hyunbo Shim, an antibody researcher at Ewha Womans University. CAR-T therapy is a T-cell therapy in which genes are recombined to destroy cancer cells by introducing a receptor gene that recognizes T cells as antigens, one of our body’s immune cells.
|
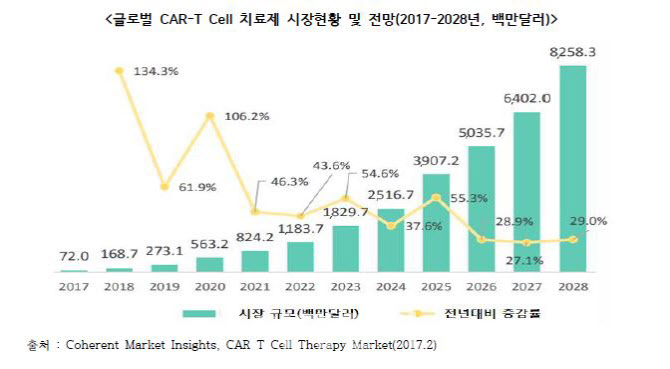
The global pharmaceutical industry is paying attention to its potential as CAR-T treatment shows a cure level of therapeutic effect in patients with leukemia, lymphoma, and multiple myeloma who do not work with existing anticancer drugs with a single administration. The global market for CAR-T treatment is also expected to expand rapidly. According to the Center for Biotechnology Policy Research, the global CAR-T treatment market is expected to grow by 54% annually from 72 million dollars (about 81.5 billion won) in 2017 and expand to 8.3 billion dollars (about 9.3948 billion won) in 2028.
CAR-T treatments that have been approved by the U.S. Food and Drug Administration (FDA) so far are the world’s first CAR-T treatments, Novartis Kimria (2017), Gilead Yescata (2018), Tecartus (2020), and BMS. There are four, including Breyanji (2021). Qrocell’s CAR-T treatment’CRC01′ is expected to be commercialized in 2023. According to the company, the CAR-T treatment can be commercialized as early as 2023 as it can significantly shorten the clinical period due to the advantage that the dosage effect appears quickly.
◇The first clinical trial in Korea… “Excellent efficacy than Kimria”
In February, it was approved by the Ministry of Food and Drug Safety for a clinical trial of’CD19 CAR-T cell therapy (CRC01)’ in adult patients with relapsed or refractory giant B-cell lymphoma. This company is the first to receive clinical approval from the Ministry of Food and Drug Safety for CAR-T treatment, which is being developed by a Korean company.
Qrocell’s core technology is the’OVIS’ platform developed by itself. OVIS is a technology that significantly lowers the expression of two types of immune checkpoint receptors, unlike the existing CAR-T technology. An official of Qrocell said, “The representative immune checkpoint receptor PD-1 is involved in the decline of immune function. Existing CAR-T treatment is a mechanism that blocks the PD-1 function with antibody treatment,” he said. “On the other hand, the treatment applied with OVIS technology can overcome the limitations of existing treatments by simultaneously removing the immune checkpoint receptor called TIGIT in addition to PD-1 to increase efficacy have. It was also confirmed that the effect of the preclinical model was significantly improved compared to the Kimlia-like model.”
|
◇ Samsung and VC industry pay attention to technology
The source of QROCELL CAR-T technology is a joint work with CEO Kim Chan-hyuk. Kim developed a new CAR-T treatment technology while working at the Caliber Research Institute in the United States. Since Professor Kim joined the KAIST Department of Life Sciences in 2016, the CAR-T treatment technology developed in the United States at the time is recognized as being jointly developed by Caliber and global pharmaceutical company AbbVie. The company is conducting a two-track business that directly launches treatments in Korea with CAR-T technology jointly developed with CEO Kim, and promotes technology exports overseas.
Qrocell technology also moved Samsung Seoul Hospital. In 2019, Qrocell established a GMP manufacturing facility for the production of clinical samples for immune cell therapy with a scale of 520m2 in the Mirae Medical Center of Samsung Seoul Hospital. According to the industry, it is not easy for venture companies to enter Samsung Seoul Hospital, but it is known that Qrocell made a proposal in 2018 and accepted it by Samsung Medical Center. The company said, “At that time, there were people who desperately sympathized with the need for CAR-T treatment at Samsung Medical Center. In 2018, since its initial establishment, QROCELL was a very small company, but it was possible because of its sincerity in developing CAR-T treatments and its own technology.”
The venture capital (VC) industry also showed expectations as a number of investors such as Intervest, Mirae Asset Capital, IMM Investment, and Stick Ventures invested a total of 61 billion won through series investments from 2017 to last year. A VC official said, “Curcel CAR-T technology is considered to be a more advanced technology than existing CAR-T treatments.” If it proves its excellent efficacy in clinical trials, it will be chosen by patients with terminal cancer and it will be easy to export technology abroad. “I said.