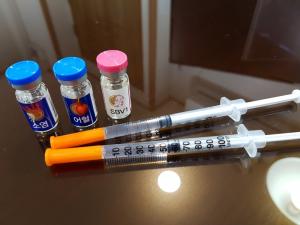
Mass manufacturing such as herbal acupuncture and neglecting to administer to the human body without permission for pharmaceutical products

[팜뉴스=김응민 기자] The Korean Pharmaceutical Association (Chairman Dae-Eup Kim) argued that immediate on-site inspection and management of the overall manufacturing and quality control of the outpatient bath room, where illegal manufacturing activities are tolerated without manufacturing standards, as well as some pharmaceutical companies that have recently become a problem, such as Binex, are required.
According to the pharmacist society, it is necessary to prepare an immediate management standard for the outpatient bath room and an immediate inspection by the relevant authorities in relation to the case where a pharmaceutical company that did not comply with the manufacturing process obligations for herbal medicines recently received a suspension of manufacturing operations.
The pharmacist society said that although the outpatient bath hall functions as a facility that mass-produces herbal medicines for a large number of unspecified people, rather than preparations according to individual prescriptions, it is in the blind spot of pharmaceutical manufacturing standards and management, threatening the health of the public.
In addition, he strongly criticized the insufficient response of the relevant authorities for avoiding proper manufacturing and quality control supervision due to the fact that it is an affiliated facility of an oriental medical institution until now, and pointed out that it is urgent to inspect the outpatient baths in accordance with the Good Manufacturing Practices Standard (GMP). to be.
In particular, the reality that mass production of injections (pharmaceutical solutions) that are injected directly into the subcutaneous or intravenously beyond the production of oral medicines in the outpatient bath room without special control such as toxicity testing is a serious situation, and the same standards as existing pharmaceutical manufacturing facilities are applied. I have to do it.
“Is it safe to take herbal medicines?”, vice-chairman of the Korean Pharmaceutical Association, said, “In a situation where public doubts about’is it safe to take herbal medicine?’ Through the manufacturing process manipulation case, it is necessary to reorganize not only the quality control of oriental medicines but also the active management and supervision system for the outpatient bath room.”
Copyright © Farm News Unauthorized reproduction and redistribution prohibited