|
[이데일리 김호준 기자] “We had the technology to make a syringe, but it was impossible without Samsung’s help from mass production to US FDA approval.
Poonglim Pharmatech’s Vice President Mihee Cho of Poonglim Pharmatech, who is planning to develop a minimum residual amount (LDS or LDV) syringe for corona 19 vaccination, and export it to the world, met with E-Daily on the 19th and explained the relationship with Samsung.
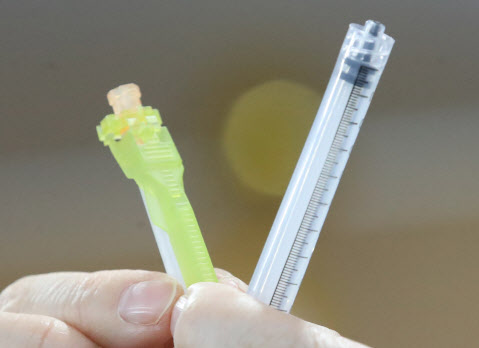
Poonglim Pharmatech’s LDV syringe minimizes the amount of residual waste left in the syringe when the vaccine is administered. The remaining amount of injection per serving (person) is 84 microliters (μL) or more for a general syringe, but 25 μL or less for an LDV syringe. In particular, Poonglim Pharmatech’s LDV syringe reduced it to 4 μL. With a regular syringe, you can only inoculate up to 5 doses per bottle of corona vaccine, but with Poonglim’s vaccine syringe, more than 6 doses per bottle are possible. It is a syringe that causes the’magic’ that increases vaccine production by 20%. Several vaccine companies, including Pfizer, have paid attention to this point and have been trying to see if they can supply syringes to Poonglim Pharmatech.
It was Samsung who visited Poonglim Pharmatech, which has the technology of making LDV syringes. Vice President Jo said, “Last year, I was informed by Samsung Bioepis if it was possible to manufacture LDV syringes. Since we had product design and patents, we established a smart factory system with Samsung Electronics and started mass production system immediately.” Said.
Since then, Samsung Electronics supported the production of prototype molds for the production of vaccine syringes through its partner factories in Gumi and Gwangju, and completed this within four days of the holiday season at the end of last year. About 30 Samsung Electronics’ manufacturing experts dispatched to Poonglim Pharmatech helped streamline the entire production process from order receipt to shipment, from syringe injection productivity to automated assembly, raw material classification management, and logistics optimization.
Vice President Cho said, “In a situation where there is a lack of technical manpower, Samsung Electronics Smart Factory Center officials directly entered the line and worked together day and night,” and “I spent time at the factory without the Lunar New Year holiday even though it was not my company’s work.” Marked.
Even with FDA approval, Samsung’s help was decisive, Vice President Cho explained. Samsung Biologics and Samsung Bioepis responded to the requirements of the FDA from the submission of documents to the FDA formal approval process, which usually takes several months or more. Thanks to this, Poonglim Pharmatech’s LDV syringe was able to obtain FDA official approval within a month.
Vice President Cho said, “Samsung Bioepis reviewed the documents to be submitted to the FDA, and carefully took care of what was lacking.” “Samsung Electronics shared the contents in real time to see if there were any special issues related to FDA approval in the US. He said that he reduced the amount.