 |
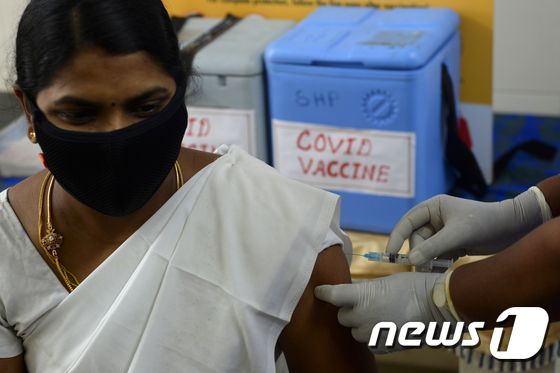
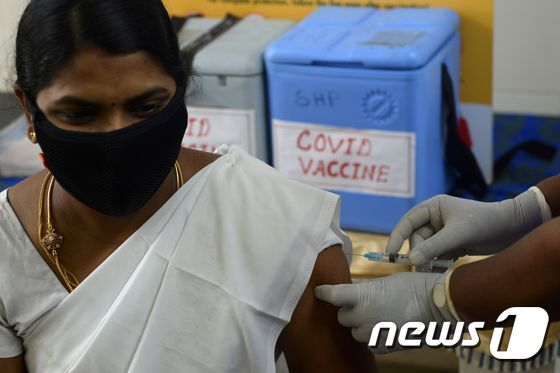
| On the 2nd, medical staff at a public health center in Chennai, India, are participating in a simulation training for the Corona 19 vaccine. © AFP=News1 |
India has approved a COVID-19 vaccine jointly developed by British pharmaceutical company AstraZeneca and Oxford University, Reuters reported on the 2nd.
According to reports, India’s Minister of Information and Communication Prakash Javdekar announced at the ruling party’s Indian People’s Party (BJP, Baratiya Zanatha Party) briefing that “AstraZeneca vaccine produced by the Indian Serum Institute (SII) was approved on the 1st.”
This gives India the first COVID-19 vaccine. However, Indian pharmaceutical regulatory authorities have not yet officially announced approval of the vaccine.
Prior to India, the UK, Argentina and El Salvador approved the AstraZeneca vaccine.
“At least three vaccines are waiting to be approved by the authorities,” said Minister Javdekar. “India is the only country with four vaccine candidates.”
Bloomberg said, “India, the world’s second-largest country, has taken its first steps in a vaccination project.” In India, there were 10.3 million confirmed cases by the 2nd. About 149,000 people died.
India plans to give priority to 300 million people out of the total population of 1.35 billion by the first half of this year. India is conducting vaccination mock training across the country with the aim of being the first vaccination next week.
However, given the large territory, limited infrastructure and poor health system, vaccination can be difficult, Bloomberg said.
AstraZeneca vaccine can be stored and transported at a normal refrigerator temperature of 2 to 8 degrees Celsius, while Pfizer must maintain a cryogenic temperature of -70 degrees Celsius. The price is also cheap. For this reason, it is expected that more AstraZeneca vaccine will be used in India.
SII has agreed to produce at least 1 billion doses of the AstraZeneca vaccine, and has already stocked 50 million doses. However, the preventive effect is about 62%, which is lower than that of Pfizer (95%).