Hanmi Pharmaceutical(369,000 -0.67%)In the fourth quarter of last year, it achieved a surprising performance (earning surprise). There is also expectation that new drugs for technology transfer will be approved by the US Food and Drug Administration (FDA) within the year.
On the 5th, Hana Financial Investment set the target price of Hanmi Pharm at KRW 430,000, KTB Investment & Securities(3,950 +3.67%)Was raised to 360,000 won. Shinyoung Securities raised it to 420,000 won. The value of new drugs scheduled for approval within this year and the recovery of Beijing Hanmi Pharmaceutical’s earnings were reflected.
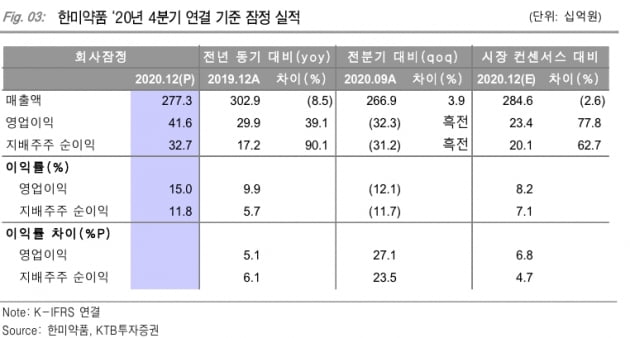
Hanmi Pharm recorded sales of 277.3 billion won and operating profit of 41.6 billion won based on consolidated financial statements in the fourth quarter. Compared to the same period of the previous year, sales decreased by 8.5% and operating profit increased by 39.2%. Operating profit exceeded the market forecast (consensus) of 23.4 billion won by 77.8%. It increased as the cost ratio improved and R&D expenses decreased.
Beijing Hanmi Pharm recorded sales of 69.4 billion won and operating profit of 19.1 billion won as China’s earnings stabilize. This is an increase of 2.4% and 65.9%, respectively, compared to the same period last year.
Hanmi Pharm’s earnings this year are expected to improve compared to the previous year. Shinyoung Securities estimates that Hanmi Pharmaceutical’s sales and operating profit will reach KRW 1,153.3 billion and KRW 95.5 billion, respectively, based on consolidated financial statements. It is expected to increase by 7.2% and 96% compared to the previous year.
Lee Myung-sun, researcher at Shinyoung Securities, said, “This year is expected to improve performance based on the fruits of the technology exports in 2015 and the base effect of last year’s results.”
Hanmi Pharmaceutical is awaiting FDA approval for three new drugs this year. The FDA market approval review date for Axol designated through the Prescription Drug Applicant Expenses Act (PDUFA) is the 28th. Rexol is an oral paclitaxel being developed by Atenex.
Pogiotinib, a treatment for non-small cell lung cancer, which has been transferred to Spectrum, is expected to submit an application for rapid approval in the first half. We look forward to approval within this year.
Spectrum is also awaiting approval for a new drug for Rolontis, a treatment for neutropenia. The PDUFA date has not yet been redesignated due to the delay in due diligence at the Pyeongtaek bio factory due to Corona 19. If the date is set, it is an observation that there is no big deal with approval.
Once Rolontis receives the final marketing approval, Hanmi Pharmaceutical can receive a staged technical fee (milestone) of 11.6 billion won from Spectrum.
Min-Jung Sun, a researcher at Hana Financial Investment, said, “In the contract with Spectrum and Artenex, the amount of ordinary technology usage fees (royalties) after the market was not known to the market.” I said.
The interpretation of Hanmi Pharmaceutical’s messenger ribonucleic acid (mRNA) vaccine consignment production (CMO) was mixed.
Researcher Lee Myung-seon said, “Hanmi Pharmaceutical is equipped with a microbial fermenter for mRNA vaccine production and an mRNA vaccine stabilization process production facility through Hanmi Fine Chemical.”
Lee Hye-rin, a researcher at KTB Investment & Securities, said, “Hanmi Pharmaceutical has no experience in the vaccine CMO business, and the competition is fierce, so it is unreasonable to be sold on stock prices. Researcher Sun said, “It will be difficult to reflect the results related to modders or vaccines during the first half of the year.”
Reporter Park In-hyuk [email protected]
Ⓒ Hankyung.com prohibits unauthorized reproduction and redistribution