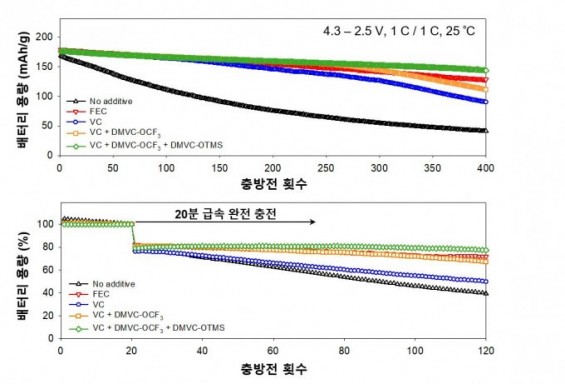
Researchers at the Ulsan Institute of Science and Technology (UNIST) successfully developed an additive for large-capacity lithium-ion batteries to maintain capacity even after hundreds of times of charging and discharging, thereby extending the lifespan. The figure is a graph comparing the capacity change during charging and discharging of batteries according to additives. When the additives newly developed by the researchers (green, orange) are included, the capacity is almost maintained even after several times of charging and discharging. Provided by UNIST
A new technology was developed in Korea that allows lithium-ion batteries to maintain 81.5% of their initial capacity even after 400 times of charging and discharging by adding a small amount of additives. As the number of times of charging and discharging the battery increases, the phenomenon of rapidly dropping from the initial capacity can be drastically reduced only by replacing the additives, raising expectations as a new method to solve the problem of battery life in large-capacity electric vehicles.
The Ulsan Institute of Science and Technology (UNIST) announced on the 14th that a joint research team including Professor Nam-Soon Choi, Professor Sang-Gyu Kwak and Professor Sung-Yoo Hong of the Department of Chemistry succeeded in extending the lifespan of lithium-ion batteries by replacing the additives used in the Department of Energy and Chemical Engineering.
Recently, in order to increase the battery capacity of lithium-ion batteries for electric vehicles, the anode material is silicon, which makes 10 times more capacity than graphite, and the anode material is actively researched to replace the high-nickel material containing 80% nickel.
However, the silicon anode has a characteristic that the volume increases by more than three times when charging and then decreases again when discharged. If charging and discharging are repeated hundreds of times, structural stress accumulates and cracks occur. This becomes a decisive factor in shortening the lifespan. High-nickel anodes containing a large amount of nickel are chemically unstable because nickel may be eluted by reacting with hydrofluoric acid (HF) contained in a trace amount in the electrolyte.
The researchers developed a new additive to be used in the electrolyte solution, a passage through which lithium ions pass, to stably protect the positive and negative electrodes of such a high-capacity lithium-ion battery while removing hydrofluoric acid.
Based on the existing vinylene carbonate (VC) structure used as an electrolyte additive, DMVC-OCF₃, an additive that can stabilize the protective film of the cathode, and DMVC-OTMS, which can remove hydrofluoric acid, were synthesized.
The researchers put these two additives in the electrolyte to make a high-capacity lithium-ion battery, and then conducted 400 charging and discharging experiments. After checking the capacity of the battery, it was confirmed that 81.5% of the initial capacity was maintained. This is a 30% increase in capacity than when using VC, an existing electrolyte additive, and 10% more than when using fluoroethylene carbonate (FEC).
In addition, even if the lithium-ion battery containing the additives developed by the research team was rapidly charged in 20 minutes and then the discharge process was repeated 100 times, the battery capacity decreased only 1.9%.
Professor Choi said, “To develop a new additive that can compensate for the shortcomings of the existing additive, VC, we first devised a synthesis method,” and said, “We presented a new direction for the development of electrolyte additives for large-capacity lithium-ion batteries.”
The research results were published on the 5th of’Nature Communications’, an international academic journal.