 |
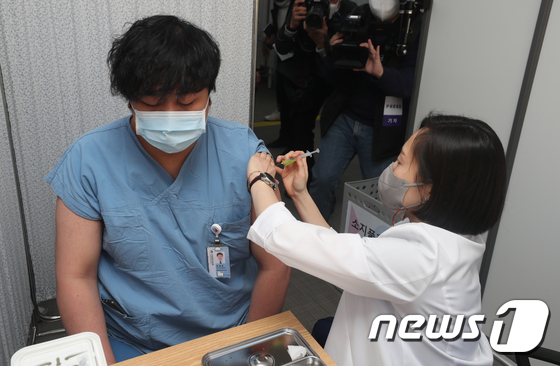
| On the morning of the 27th, a medical worker is getting a Pfizer vaccine at the Central Vaccination Center at the National Medical Center in Jung-gu, Seoul. On that day, 199 medical staff and workers at the National Medical Center receive the first dose of the Pfizer vaccine. 2021.2.27/News1 © News1 Photo Sharing Foundation |
The 3rd day of the Korean new coronavirus infection (Corona 19) vaccination reached the 28th. The first vaccination of AstraZeneca (AZ) vaccine is expected to be shipped on the same day.
The Pfizer vaccine, which targets workers in the Corona 19 treatment hospital, has also entered the second day of vaccination, and the Janssen vaccine, which can secure antibodies once it is hit, has also applied for product permission from the Ministry of Food and Drug Safety.
◇ Shipped for 1.57 million minutes on 28 days… The first vaccination is completed by the second half of next week.
According to the Corona 19 Vaccination Response Promotion Team, the first vaccination of the AstraZeneca vaccine was completed by 18,489 people (6% vaccination rate) as of 0 o’clock on the 27th. The primary target for the AstraZeneca vaccine is 280,980 people who are admitted to nursing hospitals and nursing facilities and workers under the age of 65.
According to this, the government shipped △347,000 dos on the 24th △326,000 dos on the 25th △327,000 dos on the 26th △286,000 dos on the 27th, and a total of 1.57 million dos(78) 15,000 people) will be shipped. The volume is distributed to 1,909 nursing hospitals and public health centers nationwide.
Yang Dong-gyo, head of the Resource Management Team of the Corona 19 Vaccination Response Promotion Team, said at the Corona 19 regular briefing on the 27th, “This AstraZeneca vaccine shipment is 1.57 million doses. It will be shipped by the 28th.”
Nursing hospitals can receive vaccinations according to their own vaccination plan, and nursing facilities can receive vaccinations during the holiday season according to the schedule agreed with the health center and consignment medical institution.
The AstraZeneca vaccine is expected to complete within 5 days of receipt of the vaccine, so if shipment is completed on the 28th, the first vaccination targets will be completed by the end of next week.
The AstraZeneca vaccine is administered every 8 to 12 weeks. The second inoculation will be delivered in time. Since then, the supply schedule will be decided in consideration of the quantity of COVAX facility.
◇ Day 2 of Pfizer vaccination… High school student vaccination also’green light’
In addition to the AstraZeneca vaccine, the Pfizer vaccine will be vaccinated in Korea and will begin on the 2nd day of the 28th. The estimated number of vaccinations on the 27th is 300, including 199 workers at the National Medical Center and 101 workers at the corona19 treatment hospital in the metropolitan area. The exact number of vaccination statistics will be released at 9:30 am on the 28th.
The first inoculation of Pfizer’s vaccine was Mikyung Jung, 51, who is in charge of the treatment of medical waste in the Corona 19 ward, belonging to the facility team of the National Medical Center. Jeong said, “I feel more comfortable after being beaten,” and said, “I was tense when I was hit, but it wasn’t hurting more than an intramuscular injection.”
Pfizer vaccine is administered at the Central Vaccination Center in the first week. At the same time as vaccination, visits and education are provided for medical staff at regional and regional vaccination centers. From the 2nd week (after March 3), vaccinations will be expanded to regional vaccination centers. The regional center also conducts visit training for medical staff at medical institutions that are vaccinating themselves.
The amount of Pfizer vaccine currently being vaccinated is the amount received through COVAX and has undergone a special import procedure, not an official license. Currently, the Ministry of Food and Drug Safety is undergoing formal approval review for Pfizer vaccine.
Pfizer’s vaccine has gone through the verification advisory group and the central pharmacy review committee among the three advisory bodies of the Ministry of Food and Drug Safety, and only the results of the final inspection committee remain. The Central Pharmacopoeia recommended permission for Pfizer vaccines for “age 16 or older”.
If the final inspection committee makes the same opinion as the Central Pharmacopoeia, and concludes that the Vaccination Specialist Committee of the Korea Centers for Disease Control and Prevention (KCDC), which performs vaccination, it is possible, high school students will be able to receive the COVID-19 vaccine vaccination.
“I know that the Pfizer vaccine has undergone a primary review by the Ministry of Food and Drug Safety. When the results of the approval screening come out, we plan to decide whether or not to immunize adolescents over the age of 16 through discussion of the Vaccination Committee. .
◇Once hit, Janssen’s permit screening begins… Vaccination as early as in April
In addition to the AstraZeneca and Pfizer vaccines, the quarantine authorities are also on a schedule to secure additional vaccines. The Ministry of Food and Drug Safety announced on the 27th that a multinational pharmaceutical company Janssen (a subsidiary of Johnson & Johnson) has applied for a COVID-19 vaccine.
Janssen vaccine is a’viral vector vaccine’ made by putting the corona 19 virus surface antigen gene into an adenovirus template, and was developed as a platform similar to the AstraZeneca vaccine. The Ministry of Food and Drug Safety has been preliminarily reviewing the non-clinical and quality data of Janssen vaccine since December 22 last year.
Unlike other vaccines, the Janssen vaccine is a single dose and has a prophylactic effect of 66%. The government previously announced that it would bring in 6 million Janssen vaccines in the second quarter.
The Janssen vaccination is expected to proceed within 40 days after expedited screening. Considering this, there is a possibility that it will be approved as early as March. In addition, the vaccine, which is a biological product, must undergo national shipment approval (national approval). Including this process, the Janssen vaccine is also available in April.