The competition to overcome the novel coronavirus infection (Corona 19) with vaccine development is heating up around the world. There is fierce competition for companies and countries to be recognized for their ability to develop vaccines and to increase dominance in the bio market in the post-corona era. According to the New York Times Vaccine Tracker, as of the 1st, there are 12 types of coronavirus vaccine items that are available for general vaccination in more than one country or region with urgent use approval.
Currently, 12 vaccines in the US, English, Germany, Russia, and China are released.
Market open to shortage of early-developed vaccines
Newly conducted clinical trials for 71 vaccine items
Fierce development of G7 and Korea, Australia, Israel and Japan
Already approved 4 types, try new technology vaccine
Iran, Cuba, Vietnam, and Bangladesh
Vaccine ability is set as the standard of national power
Fierce competition for post-corona bio
Vaccine technology, anti-cancer, gene therapy, etc.
Leap forward in medicine and health care through technological innovation

The Soberana 2 vaccine developed and in clinical trials by Finlay Vaccine Research Institute, Cuba This picture was taken at the Molecular Engineering Center in Havana, the capital of Cuba. AFP=Yonhap News
Corona vaccine comes out in 12 items with 5 technologies
Classified by applied technology is largely five. The mRNA technology applied by Pfizer/Bio&Tech of the US and Germany and Modena of the US was applied to vaccine development for the first time. Sputnik V (Gamalea) in Russia, AstraZeneca and Oxford University in Sweden and UK, Johnson & Johnson (J&J) in China, and Kansino in China used adenovirus delivery technology. The classic inactivation technology that kills and inoculates the coronavirus was used by Kobibaek of Russia and Barat of India, along with Sinoparm Beijing Research Institute, Sinopharm Wuhan Research Institute, and Synobac in China. As for the peptide vaccine, there is one item of Russian epibag. Developed by the United States, Germany, the United Kingdom, Sweden, Russia, China, and India, these products can be classified as mainstream vaccines in terms of the time of development and release.

On February 9th, medical workers are receiving Sputnik V coronavirus vaccine imported from Russia at Imam Khomeini Hospital in Tehran, Iran’s capital. Apart from this, Iran is the only country in the Islamic world that is developing its own vaccine. Iran has the most corona19 confirmed and dead in the Middle East. EPA=Yonhap News
Vaccine development from G7 and middle-sized countries to developing countries
Humanity’s efforts to overcome Corona 19 through vaccine development are not limited to powerful and advanced countries. When the World Health Organization (WHO), the London School of Health in the UK, the New York Times (NYT) vaccine tracker, the German international broadcaster DW, and the British Medical Journal (BMJ), a medical journal, countries around the world are competing fiercely for the development of vaccines themselves. have. In addition to the seven major countries (G7), Germany, France, Italy, Canada, and middle-sized countries, Korea, Australia, and Israel, developing countries such as Cuba, Iran, Vietnam, and Bangladesh are also joining. The lack of supply of current leaders Pfizer, Modena, and AstraZeneca, and the serious hoarding in rich countries, also opens up opportunities for latecomers of vaccines.

A Palestinian woman is being vaccinated against the coronavirus in a mobile vaccination vehicle operated by Israeli authorities near the Damascus Gate in Jerusalem on February 26th. In the background, other people are in line waiting for their turn. Israel imports and uses the Pfizer vaccine, but is also developing its own vaccine. AFP=Yonhap News
NovaVax·Curebag·ZF2001·GSK Phase 3 clinical trial
According to the New York Times (NYT) vaccine tracker on the 1st, there are 71 coronavirus vaccines currently in clinical trials, of which 20 are undergoing phase 3, the final stage. In a clinical trial that confirms the safety and effectiveness of vaccines in humans, in phase 1, the safety and required dose are investigated in small-scale subjects, in phase 2, the scale is increased to confirm safety, and in phase 3, the effectiveness is verified in large-scale test groups. do. Animal testing is a pre-clinical trial, which is to explore the potential of candidate substances through animal testing. Currently, at least 78 novel coronavirus vaccine candidates are in the animal testing phase. This is an early stage of looking at the potential of a vaccine.

Incepta’s vaccine factory in Dhaka, the capital of Bangladesh. The company is preparing for consignment production of overseas vaccines. Bangladesh is a developing country, but it has also started developing its own vaccine. AP=Yonhap News
It is expected that the 4 items undergoing phase 3 clinical trials will be the first to be vaccinated if the results are good. Among them, the NovaVax vaccine being developed by NovaVax of the United States and the ZF2001 vaccine being developed by Chinese biotechnology company Anhuijipeilongkerma applied the same technology of extraction of specific antigens. Specific antigen vaccines (subunit vaccines) are manufactured by extracting antigenic components that can cause immune function among viral components.
The CureVac vaccine, which is being developed by German biosign CureVac, uses RNA technology, and Cadillac, an Indian pharmaceutical company, is a plasma DNA vaccine that uses DNA, the blueprint that makes up the antigen, as a vaccine. In addition, the UK pharmaceutical company GlaxoSmithKline (GSK) and Canada’s Medicago are developing a recombinant vaccine using a virus-like substance, and are in simultaneous phase 2 and 3 clinical trials. This is the item that has entered a large-scale phase 3 clinical trial.
6 clinical trials approved in Korea
In Korea, two cases of SK Bioscience, Genexine, Jinwon Life Science, and Celid each have been approved by the Ministry of Food and Drug Safety for corona vaccine clinical trials. Here, the International Vaccine Research Institute (IVI), an international organization headquartered in Korea established by the United Nations Development Program (UNDP), is the first of the corona vaccine developed by the US biotechnology company Inovio with the support of the International Federation for Infectious Diseases and Innovation Phase 2 clinical trials are in progress in Korea.
![On the 17th, when the corona19 vaccination began in Japan, Kazuhiro Araki (left), director of the Tokyo Medical Center in Tokyo's Meguro-ku, was selected as the first vaccine target in Japan and is receiving an injection. Japan is also in phase 1 clinical trials after developing its own vaccine. [연합뉴스]](https://i0.wp.com/pds.joins.com/news/component/htmlphoto_mmdata/202103/02/fb7ec5f5-eb07-4a4b-898c-92df119a4684.jpg?w=560&ssl=1)
On the 17th, when the corona19 vaccination began in Japan, Kazuhiro Araki (left), director of the Tokyo Medical Center in Tokyo’s Meguro-ku, was selected as the first vaccine target in Japan and is receiving an injection. Japan is also in phase 1 clinical trials after developing its own vaccine. [연합뉴스]
The Israeli Institute of Biological Research (IIBR) is conducting phase 2 trials of IIBR-100, a recombinant vaccine. Vietnam’s Nanogen is conducting a phase 2 trial of a vaccine called Nano CoVax using a specific antigen extraction method. Korean companies have also invested.
In Japan, with the support of the Japan Medical Research and Development Organization (AMED), a national institution with a budget of 126 billion yen (about 1.3 trillion won) per year, Anses, a gene drug developer, conducted a phase 2 clinical trial of AG0301, a plasmid DNA vaccine. Is in progress.
In Italy, a pharmaceutical powerhouse, a vaccine, which is being developed jointly by biotechnology companies Takis and Rotapharm, entered a phase 1 clinical trial on February 10th. The two companies signed a vaccine joint development contract in June of last year and have been developing it, Italy’s ANSA news agency reported.

On February 16, Sinoparm vaccine was loaded in a warehouse in Baijing, China. Reuters = Yonhap News
China has already approved 4 types of advanced vaccine development
China is drawing attention. On December 31 of last year, China gave conditional approval to the Sinoparm (China Pharmaceutical Group) Beijing Research Institute vaccine, and on February 6, the SinoVac vaccine. On February 25, Sinopharm’s Wuhan Bioproduct Research Institute and Kansino Bio had conditionally approved the general use of the applied vaccine. This will allow China to receive all four coronavirus vaccines. Cancino products are carrier vaccines that utilize adenovirus, and the remaining three are inactivated vaccines that kill and inoculate novel coronaviruses.
In addition, China is focusing on the development of vaccines using advanced biotechnology. In addition to ZF2001, which applied specific antigen extraction technology, the People’s Liberation Army is developing an RNA vaccine with Wallbox Biotech. Other biotech companies are developing two vaccines using carrier technology. On February 18, Shenzhen’s Kangtai Biologic Products entered a phase 2 clinical trial of the vaccine, NYT reported.

A worker is getting a vaccine on February 9 at Imam Khomeini Hospital in Tehran, Iran’s capital. Shinhwa = Yonhap News
Iran is developing vaccines in the Islamic world
Iran, which has the largest number of corona19 confirmed and dead in the Middle East, is also spurring vaccine development, German international broadcaster DW reported. DW said more than 1.4 million confirmed cases and more than 58,500 deaths have occurred in Iran so far.
According to the Associated Press and the New York Times, Iran started a phase 1 clinical trial of its own coronavirus vaccine candidate on December 29 last year, and on February 7, the second candidate also entered a phase 1 clinical trial. The vaccine candidate was developed by Sifa Parmed, a subsidiary of Iran’s state-run pharmaceutical company Barrecat. The company has been producing antibiotics, including penicillin. Al-Jazeera broadcaster reported on February 4 that Iran had decided to introduce 10,000 doses of Russia’s Gamaleya (Sputnik V) vaccine first. It seems that it is on the two tracks of overseas introduction and self-development at the same time.

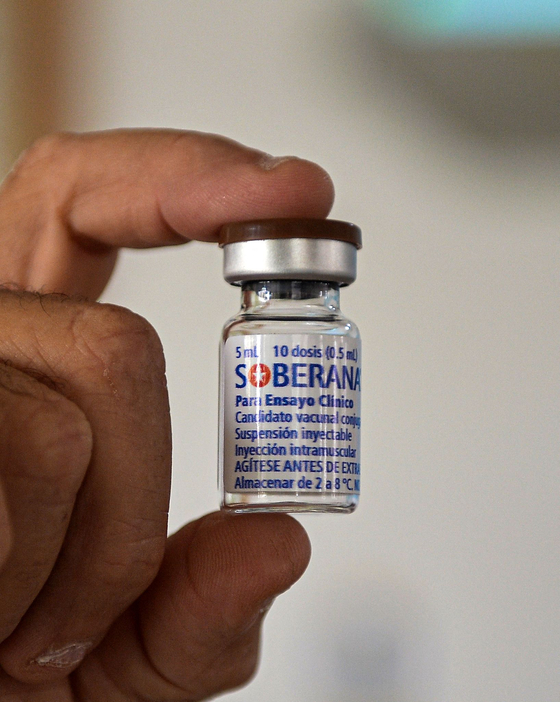
On February 25, at the Center for Genetic Engineering and Biotechnology in Havana, the capital of Cuba, a scientist is conducting an experiment on Abdala, an experimental coronavirus vaccine developed by Cuba. AP=Yonhap News
Cuba also developed a vaccine for phase 3 clinical trials
The Abdala vaccine (CIGB66), which is being developed by the Cuba’s Center for Genetic Engineering and Biotechnology (CIGB), entered a phase 2 clinical trial on February 1, the medical journal British Medical Journal (BMJ) released the following day. BMJ released a letter from the Cuban authorities, according to which the vaccine candidate began phase 1 clinical trials on December 7 last year, and as a result of this, it was found that the immune substance was expressed smoothly when two doses of it were vaccinated. As a result, the letter said it had entered a phase 3 clinical trial at the Saturnino Lora Hospital in Santiago de Cuba.

On March 1 at a hospital in Bangalore, India, a worker is vaccinated with the home-made AstraZeneca vaccine. India is developing its own coronavirus vaccine that is sprayed in the nose. AFP=Yonhap News
In India, a vaccine for nasal spray is being developed.
In India, two nasal spray vaccines, which are administered by spraying drugs into the nose instead of injection, are undergoing phase 1 clinical trials. The Indian daily Times of India reported on January 12 that the world’s largest vaccine producer, the Indian Serum Research Institute (SII), entered a phase 1 clinical trial of a nasal spray vaccine jointly developed with biotechnology company Kodagenix. On February 16, Barat Biotech said it had begun a phase 1 clinical trial of another nasal spray vaccine.
In Bangladesh, a phase 1 clinical trial of BangaVax, developed by Globe Biotech, is in progress. Bangladesh has a per capita gross domestic product (GDP) of $1888, Vietnam’s $3498 and Iran’s $7257, based on an estimate of the nominal value of the International Monetary Fund (IMF) in 2020. Cuba’s World Bank (WB) statistics for 2018 cost $8822. It is a case of showing that vaccine development is not exclusive to rich countries, only with a difference in speed.
![The actual appearance of the novel coronavirus, taken by the National Institutes of Health (NIH). [AP=연합뉴스]](https://i0.wp.com/pds.joins.com/news/component/htmlphoto_mmdata/202103/02/f68c87a4-eb70-42c7-8481-fffcf3a930b4.jpg?w=560&ssl=1)
The actual appearance of the novel coronavirus, taken by the National Institutes of Health (NIH). [AP=연합뉴스]
Post Corona Bio Competition Begins
Amid the corona crisis, it shows a willingness to accumulate technology, marketing experience and know-how through vaccine development, and to aim for the bio market in the post-corona era by raising the image of companies and national brands. The technologies such as mRNA, adenovirus carrier, specific antigen extraction, and plasmid DNA applied in the development of the corona vaccine can be applied to various uses including anticancer drugs and gene therapy as well as infectious diseases in the future. It is to use the gloomy era caused by the corona as an opportunity to open the era of health and longevity through the leap of biotechnology. Biotechnology and vaccine capabilities are expected to play an important role in international relations in the future.
In-Taek Chae International Reporter [email protected]
![]()