Enter 2021.01.27 14:50 | Revision 2021.01.27 16:15
Expert “Preparing for Irritable Shock Epinephrine Need to hold”
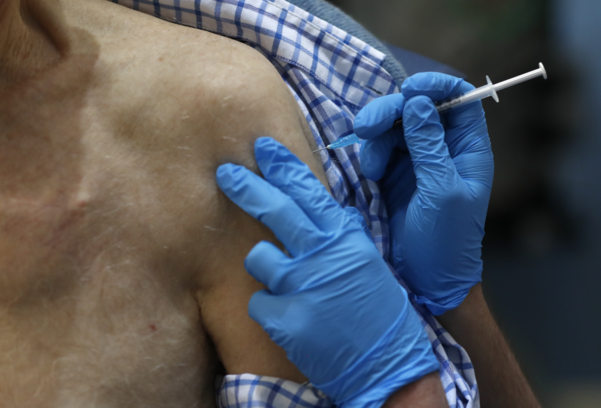
The government announces detailed plans for corona vaccination on the 29th

The Center for Disease Control and Prevention (CDC) reported that at least 29 of the 5.3 million people who received the Corona 19 vaccine had suffered anaphylaxis through an official media briefing in the United States on the 6th (local time), the Associated Press and The Wall Street Journal reported. This is 4.2 times that of the influenza vaccine, which amounts to 5.5 or 1.3 per million. These 29 people were vaccinated with vaccines manufactured by Pfizer, Bioentech or Modena. Of the 1.9 million Pfizer vaccinations, 21 suffered from anaphylaxis, acute hypersensitivity.
Currently, the government has signed vaccine purchase contracts with four pharmaceutical companies such as Kovacs Facility, AstraZeneca, Janssen, Pfizer, and Modena, which are international projects, securing a total of 56 million people. In addition, it has almost completed a purchase contract for 20 million people with Nova Bax.
It is observed that Korea will also start vaccination from next month, and if there is a history of severe allergic reactions, countermeasures are expected.
Common side effects appear in cases of COVID-19 vaccination in the US and UK. Pfizer, Modena, and AstraZeneca vaccine phase 3 clinical results show pain, itching, swelling, and skin redness (irritating red swelling) at the vaccination site. It is a local side effect that manifests in any one of the body parts. Systemic side effects such as chills, muscle pain, tiredness, and headache also appear.
As a result, experts explained that side effects or long-term complications that may appear after vaccination against Corona 19 should be considered. Park Wan-beom, a professor of infectious medicine at Seoul National University Hospital, said, “Both Pfizer, Moder and RNA vaccines can cause similar side effects to other vaccines.” Local reactions such as pain, redness, and swelling can occur,” he explained. Both Pfizer and Modena vaccines found that vaccine side effects occurred mainly after the second dose. In the case of the Pfizer vaccine, 16% complained of fever, 26% of headaches, 38% of muscle pain after the second vaccination, and Modena vaccine was similar. Prof. Wanbeom Park said, “Long-term complications have not yet been reported, but since RNA vaccine is a new type of vaccine, follow-up studies are needed for long-term complications.”
In particular, anaphylactic reactions with lowering blood pressure or respiratory failure may also appear infrequently. Anaphylaxis refers to a severe allergic reaction that occurs systemically within minutes and hours after exposure to a causative agent such as certain foods and drugs. In the fall, wasps sting and death often occur. The cause of death from bongchim therapy is anaphylaxis. As such, when an allergy caused by a specific antigen occurs, symptoms such as shortness of breath due to anaphylactic shock may occur, leading to death in severe cases.
Accordingly, there is an opinion that each inoculation hospital should be prepared for serious side effects such as anaphylaxis. “Corona 19 vaccination is necessary, and the way we should go,” said Cho Seok-ju, a professor of emergency medicine at Pusan National University Hospital. Said.
At the same time, it was essential to have emergency medications for each hospital to prepare for the outbreak of anaphylaxis. Professor Seok-joo Cho said, “For the treatment of anaphylaxis, foreign countries are taking epinephrine, a first-aid medication that is also prescribed for self-injection. However, it is highly likely that some hospitals and clinics in Korea do not have epinephrine. All hospitals and clinics that inject the vaccine have this drug. It is necessary to prepare and retrain the indications and usage.” He also said, “One or two for each medical institution would be sufficient.”
Some are afraid of the side effects of the COVID-19 vaccine and are trying to avoid vaccination. Experts recommended vaccination, as it can be seen that the chances of getting infected with the Corona 19 virus can be reduced to one in 20 if vaccinated against the Corona 19 vaccine. The benefits of vaccination are greater than the side effects of the vaccine. CDC explained that the side effects of the Corona 19 vaccine are similar to other vaccines, and start within the day or two of the vaccination and disappear after 2-3 days.
The government will announce details through the corona19 vaccination implementation plan to be announced on the 28th. The vaccination plan includes the vaccination target, vaccination institution, implementation standards, and post vaccination adverse reaction management system.