 |
| © News1 Designer Kim Il-hwan |
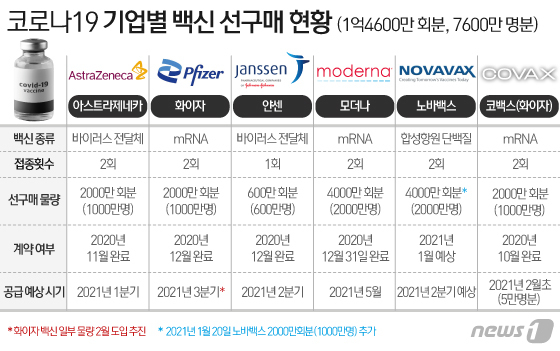
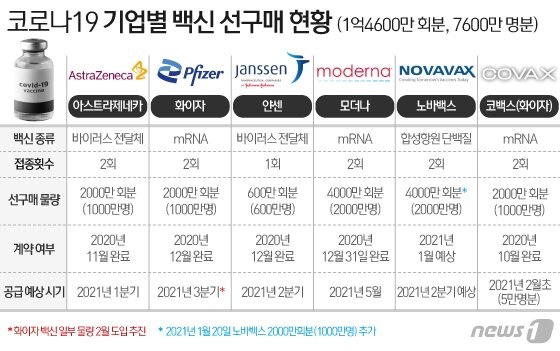
Next month, the Pfizer-AstraZeneca vaccine, 50,000 plus (+) alpha (α), is expected to be the first vaccine for medical staff. Vaccination is expected to begin as early as mid-February. The government plans to accelerate the vaccination time and increase the amount of vaccination as much as possible, with the goal of completing the national vaccination by the third quarter. The outline is emerging ahead of the government’s announcement of the’Corona 19′ vaccination plan at the end of this month.
The amount of vaccine that can be vaccinated in the first quarter of this year is likely to be insignificant compared to the entire nation, considering the time of introduction and preparation process in Korea. Since a large number of them will be introduced in Korea in the second and third quarters, the actual vaccination of nearly 50 million people is expected to be concentrated during this period.
◇Vaccination seems to be possible from mid-February as soon as possible… Intensive vaccination in the 2nd~3rd quarter
According to the government on the 23rd, a vaccine equivalent to 50,000 vaccinations will be introduced for the first time in Korea from COVAX facility, a global vaccine co-purchase alliance early next month. While Kovacs has made such a proposal to the government, it is expected that the government will accept the vaccine as soon as possible. The quarantine authorities explained that the final decision will be made at the end of this month. The COVAX vaccine is selected from Pfizer and AstraZeneca and GSK-Sanofi vaccines. Among them, it is known that the Pfizer vaccine was decided.
In order for Pfizer vaccine to enter Korea in early February, it must first obtain an item permission from the Ministry of Food and Drug Safety. The Ministry of Food and Drug Safety has shortened the review period to less than 40 days from application to approval, and it is expected that the application for Pfizer vaccine approval will be made soon considering the timing of introduction.
As Pfizer’s vaccine was first approved in the US and Europe, the domestic approval review period may be earlier than expected. However, since it was introduced in early February, it is necessary to go through the procedure for national shipment approval (national inspection) by the Ministry of Food and Drug Safety. The national inspection review usually takes 2 to 3 months, but the Ministry of Food and Drug Safety has set up a professional team for this Corona 19 vaccine and treatment, and has made plans to complete it within 20 days. Therefore, the timing of physical vaccination is expected to be after mid-February as early as possible.
The government plans to introduce some of the AstraZeneca vaccine, which was previously planned for inoculation at the end of February, in order from February. In this case, two types of vaccines will be available within February, even to the Pfizer vaccine. AstraZeneca has already applied for permission from the Ministry of Food and Drug Safety and is undergoing examination.
The government previously signed contracts with each company to bring in 20 million modal vaccines in May, 6 million Janssen vaccines in the second quarter, and 10 million vaccines separately from Pfizer in the third quarter. In addition, the introduction of 20 million NovaVax vaccines is almost certain. NovaVax vaccine is likely to be introduced in the second to third quarters as it has not yet completed phase 3 clinical trials.
In fact, the time to enter Korea is concentrated in the second and third quarters. The government aims to complete the nationwide vaccination by the third quarter of this year and to generate a’collective immunity’ that suppresses the possibility of spontaneous transmission of infection by November. The difference of about a month takes into account the period of immunity after the vaccination is completed.
 |
| © News1 Designer Kim Il-hwan |
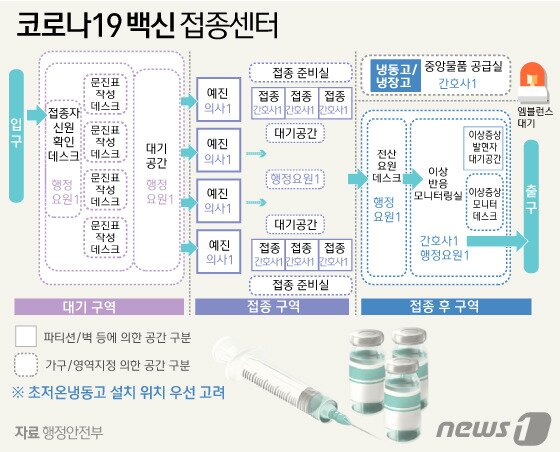
◇ Establish 250 vaccination centers that need low temperature storage
The government is running a cross-ministerial corona 19 vaccination response promotion group centered on the Korea Centers for Disease Control and Prevention. The Ministry of Land, Transport and Maritime Affairs is responsible for the transfer of vaccines from overseas to Korea, the Ministry of Defense for domestic transportation, and the Ministry of Trade, Industry and Energy for supply and demand of cryogenic freezers. The Ministry of Public Administration and Security, together with local governments, is in charge of organizing vaccination promotion teams and regional councils, and selecting consigned medical institutions and vaccination center candidates.
Pfizer vaccines and modderna vaccines are made based on genetic mRNA and must be stored and transported in a cryogenic environment (minus 20 to 70 degrees Celsius). Therefore, the government is establishing a separate vaccination center for these vaccinations.
The Ministry of Public Administration and Security plans to operate 250 vaccination centers, and 150 sites were selected as of 6 pm on the 20th. The rest will be confirmed early next week. One or more vaccination centers are designated per city, county, or district, and about three if the population exceeds 500,000. Facilities such as indoor gymnasium, performance and cultural facilities, idle areas, sports grounds, parks, and hospitals are applicable. In case of sudden side effects, emergency medical institutions should also be close. If there is not enough manpower and space in the area in charge, it will be jointly installed with nearby municipalities.
The rest of the vaccines can be vaccinated at local clinic-level designated medical institutions as they can be distributed at low temperatures, similar to the existing flu vaccine. The vaccination institution is expected to be received by the Korea Disease Administration.
The government initially selected elderly people in nursing hospital facilities as the first candidates for vaccination, but recently turned to medical staff. This is because Pfizer vaccination deaths among elderly people in some countries, such as Norway, are one after another. However, the Norwegian public health agency said, “It is difficult to say that the deaths were related to the vaccine, as 45 people die on average per day in nursing homes in Norway.” The quarantine authorities are in the position that it is necessary to review whether or not to immunize the very elderly.
The priority vaccination targets set by the government are ΔMedical institution workers ΔGroup facility residents and workers ΔElderly people aged 65 or older ΔAdult chronically ill ΔChildren and youth education and childcare facility workers and employees ΔCorona 19 primary response personnel ΔAdults aged 50 to 64 ΔPolice Firefighting civil servants and military personnel Δ Correctional facilities and treatment center prisoners and staff. In general, people who are vulnerable to Corona 19 or human resources essential to society are the targets. The remaining 19-49 years old healthy adults start vaccination from the third quarter.
 |
| SK Bioscience’s vaccine factory Andong L House. © News1 |
◇ Selected as’SK Bioscience’ as a vaccine storage and distribution agency
The quarantine authorities selected SK Bioscience as a company dedicated to establishing a distribution management system for vaccines. SK Bioscience, which has already decided to take over the production of AstraZeneca and NovaVax vaccines, has been selected as a storage and distribution agency to transport the vaccine to the inoculation center.
SK Bioscience plans to operate a system that can monitor temperature maintenance and delivery routes in real time during vaccine transportation by establishing a customized cold chain (low temperature distribution) management system for each vaccine.
To this end, M2Cloud will participate as a partner and build an integrated control center based on the Internet of Things (IoT) to create a real-time temperature management and vaccine location tracking system.
SK Bioscience plans to establish a refrigerated and refrigerated distribution center by establishing a cooperation system with domestic logistics companies to prepare for the uncertainty of the vaccine supply timing and the liquidity of the vaccine supply. Through this, the system will be equipped with a system that can manage the status of vaccination supply by region and by vaccination agency, as well as management of stock and receipt by vaccine. Gtree B&T and Dongwon I-Farm will participate as distribution partners. Dongwon I-Farm is responsible for securing a cryogenic distribution warehouse and building a distribution center for vaccine storage at -75 degrees Celsius.
In addition, the Korea Centers for Disease Control and Prevention is preparing to install a’cryogenic freezer’ in the vaccination center, an essential item for maintaining the Pfizer vaccine cold chain. The disease administration provides government subsidies (about 250 units) to companies registered in the Nara Marketplace (Daehan Science, Ilshin Biobase, Thermo Fisher Scientific) in each local government to purchase a cryogenic freezer, and each local government designates an inoculation center. And the freezer purchase and installation preparations will proceed.
Commissioner Jeong Eun-kyung said, “Establishing a thorough distribution management system for vaccines is the most important task for safe vaccination,” and “We will prepare carefully and without disruption until vaccination is implemented.”