With the government proclaiming to invest 4 trillion won per year in national biohealth research and development (R&D) in 2019 by 2025, the investment strategy in the field was completed in about two years.
The Ministry of Science and ICT’s Science and Technology Innovation Headquarters announced on the 29th that it had deliberated and confirmed the’Bio Health R&D Investment Strategy 2’at the level of the ministry at the 9th Bio-Special Committee.
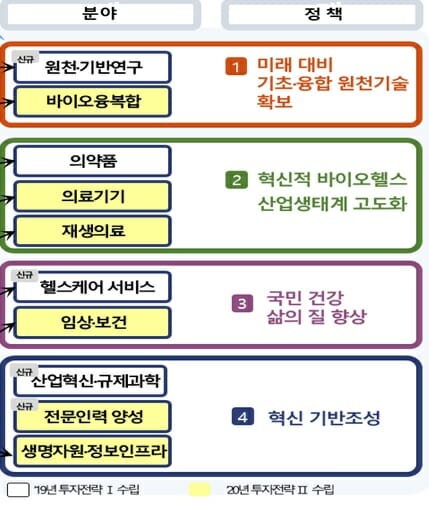
The investment strategy 2 established this time follows the investment strategy 1 established by the Biospecialty Commission in December 2019, and 4 of the 10 areas linked to technology and bio policies at the time (▲ source and base research ▲ medicine ▲ healthcare service) ▲Industrial innovation and regulatory science) investment strategies were first prepared. In this investment strategy 2, strategies for the remaining six areas, such as ▲ bioconvergence ▲ medical devices ▲ regenerative medicine ▲ clinical and health ▲ professional manpower training ▲ life resources and information infrastructure, are included.
In May 2019, the government announced that it would expand the government’s R&D investment in the bio-health sector, worth 2.6 trillion won per year, to more than 4 trillion won by 2025. In order to support the development of domestic new drugs with annual sales of more than 1 trillion won, it decided to invest more than 2 trillion won in policy financing in the bio-health field over the next five years using the’Scale Up Fund’, which is being created with a total of 15 trillion won by 2022.
The Science and Technology Innovation Headquarters organized and operated a working group in which a total of 90 industry, academia, and research experts participated to establish this investment strategy and listened to the voices of the field.
Seong-soo Kim, head of the Science and Technology Innovation Division, said, “As the public’s interest in health and medical care increased due to the Corona 19 pandemic, it became an opportunity to examine domestic biohealth research and industrial capabilities. “This strategy is planned to be used in the’National R&D Investment Direction and Standards’ in the bio-health field, so the relevant ministries that are promoting the project will actively seek out blank areas and promote new business plans by referring to this strategy. I asked.
■Source/Base Research
It was intended to strengthen support for linkage research that can advance basic research results such as thesis and patents into source technologies that can be used in the biohealth field. In particular, it expands support for discovering new targets for the investigation of disease mechanisms and overcoming diseases, and provides mid- to long-term support for universal new concept and new technology R&D in biohealth research that can become a leading research country out of pursuit research.
■Bio Convergence
We will expand investment in R&D, the source of next-generation drug development, such as improving the efficiency of new drug development and improving drug delivery technology through convergence with advanced technologies such as artificial intelligence. Support will be reinforced to secure advanced convergence medical device technology and original materials and parts technology for strengthening global competitiveness and linking to the medical device industry.
■Medicines
It focuses on the verification of new core targets and the discovery of initial pipelines. We support the development of innovative technologies such as the source technology of next-generation high-tech medicines, and provide mid- to long-term support to strengthen full-cycle capabilities from discovery of candidate substances to commercialization. In addition, it supports the establishment of a common infrastructure (AI, etc.) platform for the development of evaluation technologies for emerging therapeutic fields and promotion of new drug development.

■Medical equipment
Reinforce customized R&D support such as localization and future medical-leading strategies through linking the government’s major policies and budget. In order to commercialize research results and ideas, and to preempt international medical device standards, the company will strengthen domestic medical device technology commercialization capabilities and support R&D based on overseas expansion.
■Advanced regenerative medicine
We will continue to support intermediary research to secure original technologies in the field of next-generation regenerative medicine, such as general-purpose stem cells, and to secure therapeutics based on original research results. In addition, it will strengthen mid- to long-term support for R&D to enhance the functionality of regenerative medical treatments such as applying gene editing technology and diversify treatment pipelines. We will discover R&D tasks for improving industrial utilization of regenerative medicine-derived technologies such as stem cell and organoid-based disease modeling and commercialization of technologies related to regenerative medicine front and rear industries.
■Healthcare service
We support the development of common platform technologies such as healthcare big data standardization and security technologies that can lead to social consensus within the scope of related regulations. Support for empirical research for utilization of healthcare and activation of services, support for core technology development in new technology areas, and discover and support areas for improving the quality of life of the people.
■Clinical/Health
To reinforce the linkage of basic research results to clinical and practical use, we will continue to support intermediary research for public interest such as diseases attributable to living environments and habits, mental diseases, and rare diseases. We will strengthen support for public-interest clinical research to establish the basis for RWE production and use and to promote clinical application of new treatments. In addition, we will continue to invest in basic and basic research and development of infectious diseases, such as developing predictive modeling, securing core platform technology for new concept diagnosis, treatment, and vaccines, and researching the mechanisms of various pathogens.
■Industrial innovation and regulatory science
In order to promote startups, technology commercialization, and overseas expansion, we continue to invest to lay the groundwork for infrastructure and cooperation systems such as empirical research space. From the R&D stage, we continue to support research to improve licensing and regulations, including support for reviewing technology-regulation consistency
■Cultivation of professional manpower
Reinforce new investments in promising future fields (convergence of BT + artificial intelligence, etc.) technology convergence and health care (basic medical scientists, physician scientists, etc.) manpower training projects. In addition, it supports process development and training of regulatory science experts for commercialization of research results, and discovers and supports the establishment of an industrial demand-based manpower training-recruitment linkage program and a demand-supply and future demand forecasting platform to revitalize the use of experts and lay the foundation. do.
Related Articles

GNC Energy to supply emergency gas turbine generator to Samsung Biologics

Koh Han-seung, CEO of Samsang Bioepis, inaugurated as the new President of Korea Bio Association

Seoul Bio, mass production of 25Gbps big cells… targeting the 1.2 trillion market

Anticancer substance developed by Samsung Biologics, approved by FDA for Phase 1 and 2 clinical trials
■Life research resources and information infrastructure
Reinforce R&D investments to preemptively secure bio-research resources with high research utilization and industrial impact in the bio-health field and advance resources. We continue to support R&D for self-sufficiency of research resources on the basis of strengthening the capabilities of advanced technology to utilize life research resources (materials + information).