Effective as a treatment to recover symptoms such as fever and cough
The Ministry of Food and Drug Safety recommends approval of preliminary products for phase 3 clinical trials.
The 2nd and 3rd verification, such as the time required and safety, should be thorough
[서울=뉴스핌] Reporter Seo Young-wook = The interest in Celltrion’s novel coronavirus infection (Corona 19) antibody treatment,’Recyronaju (ingredient name Legdanvimab, developed name CT-P59)’ is hot. The Ministry of Food and Drug Safety confirmed the safety and efficacy of Celltrion’s Phase 2 clinical results.
As the verification advisory group recommended product approval on the premise of conducting phase 3 clinical trials, the possibility of field injection as early as early next month increased. However, experts point out that due to the limited amount of publicly available data, a thorough verification work must be carried out before entering the field.
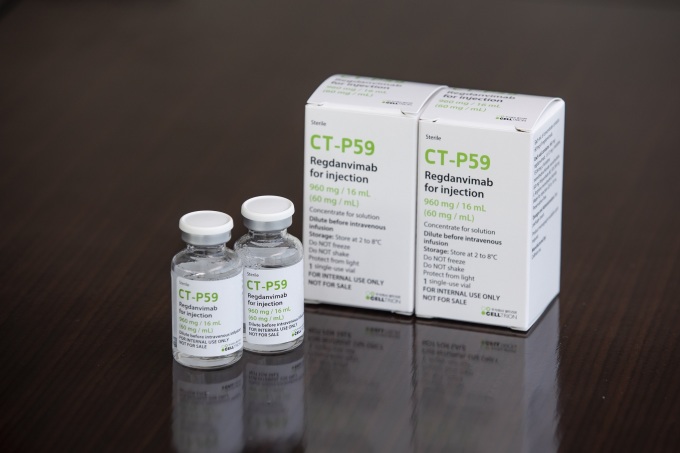
 |
| Celltrion’s COVID-19 treatment, Rekironaju [제공=셀트리온] |
Shortened recovery period for symptoms such as lekkironaju, fever and cough
Celltrion’s treatment should be focused on shortening the recovery period, shortening the time it takes to switch from positive to negative during virus testing, and so-called’shortening the time required for negative conversion.’
Looking at the results of Celltrion’s own results and the first verification (verification advisory meeting) of the Ministry of Food and Drug Safety, half of the purpose was achieved.
First of all, the verification advisory group confirmed that the recovery period of the bequest box is shortening.
According to the Ministry of Food and Drug Safety, the verification advisory group has been observing the intensity of symptoms by administering Rekironaju and placebo (a fake drug) twice a day for 14 days to patients who show any symptoms of Corona 19 such as fever.
The main symptoms of Corona 19 are ▲ fever ▲ cough ▲ difficulty breathing ▲ sore throat ▲ whole body pain (muscle pain) ▲ fatigue ▲ headache.
In the case of patients who received 40 mg of Rekirona per 1 kg of body weight, it took 5.34 days until all of the 7 symptoms were judged to disappear or weaken.
Conversely, it took 8.77 days for patients who took placebo. Patients who received Rekirona injection were 3.43 days earlier.
The verification advisory group said, “It is statistically’significant’ that the time for improvement of Corona 19 symptoms has decreased, which is clinically significant.”
However, it is not yet known whether taking this drug will accelerate the discharge of patients in the hospital.
The Ministry of Food and Drug Safety explained, “It is difficult to say whether the improvement of symptoms will lead to shortening the discharge time.”
◆’Reduction of time required for negative transfer’ is concluded in the 2nd and 3rd verification
Unlike the reduction of the recovery period, the conclusion on the reduction of the negative pre-transition time was actually postponed to the 2nd and 3rd verification.
The verification advisory group explained, “There was no significant difference in the time between the positive to negative virus test results between the patients taking the drug and those who did not,” he said. “After administration of this drug, a tendency to decrease the virus concentration in the body was observed.”
He added, “There is a limitation of the test method itself that the virus measurement method is not standardized and there is a large deviation between the test results, so the result of the time required for virus negative transduction is not clinically significant.”
The Ministry of Food and Drug Safety said, “The Central Pharmacopoeia and the Final Inspection Committee will discuss the negative results of the virus once again and make a comprehensive judgment.”
Nevertheless, as the verification advisory group recommends that the product be approved on the premise of conducting phase 3 clinical trials, Rekkirona is expected to be used by patients soon.
Prime Minister Jeong Sye-gyun said at a meeting of the Central Disaster and Safety Countermeasures Headquarters on the 18th, “I expect that Rekkirona can be put into the field early next month.”
 |
| Corona 19 treatment vaccine approval procedure [제공=식약처] |
◆Death after vaccination abroad.. Safety must be additionally verified.
Previously, 29 elderly people with serious diseases in Norway died after receiving the vaccine, and the safety of the vaccine and treatment is also a very important item.
First of all, the verification advisory group revealed that there were no significant side effects of Rekirona.
The verification advisory group said, “There were usually mild or moderate abnormalities after administration of this drug, but the ratio was similar when compared with those who received this drug and not.” There was no” he explained.
Still, he commented that “the effect on the mortality rate cannot be known because neither patient who received this drug nor the patient who did not die.” This part also needs thorough verification through a phase 3 clinical trial.
◆Rekirona is expected to play a role as a vaccine
Although Rekirona is a therapeutic agent, Celltrion was initially considered as a vaccine.
Celltrion received approval from the Ministry of Food and Drug Safety in October last year for the clinical trial plan (IND) of Phase 3.3, which is a preventive clinical trial in Rekirona.
At that time, Celltrion was planning to conduct a preventive clinical trial of CT-P59 on a scale of 1,000 people with close contact and asymptomatic confirmation in Korea.
However, Celltrion plans to focus on developing treatments for the time being, and the schedule for preventive clinical trials is unknown.
 |
| Rekirona main development schedule [제공=셀트리온] |
◆Vaccines are supplied from global pharmaceutical companies
As there is currently no COVID-19 vaccine developed by a domestic pharmaceutical company, the quarantine authorities are focusing on securing a vaccine for global pharmaceutical companies.
Currently, the pharmaceutical companies that the government has signed a vaccine supply contract are Modena (for 20 million people), AstraZeneca (for 10 million people), Pfizer (for 10 million people), and Janssen (for 6 million people).
In addition, 10 million people will be supplied through the’COVAX facility’, an international project for joint purchase and distribution of vaccines, and the total amount of vaccines secured is 56 million.
Starting with the AstraZeneca vaccine in the first quarter, vaccines from global pharmaceutical companies will be introduced in Korea. The timing of supply of COVAX vaccine is expected to be confirmed at the end of this month.