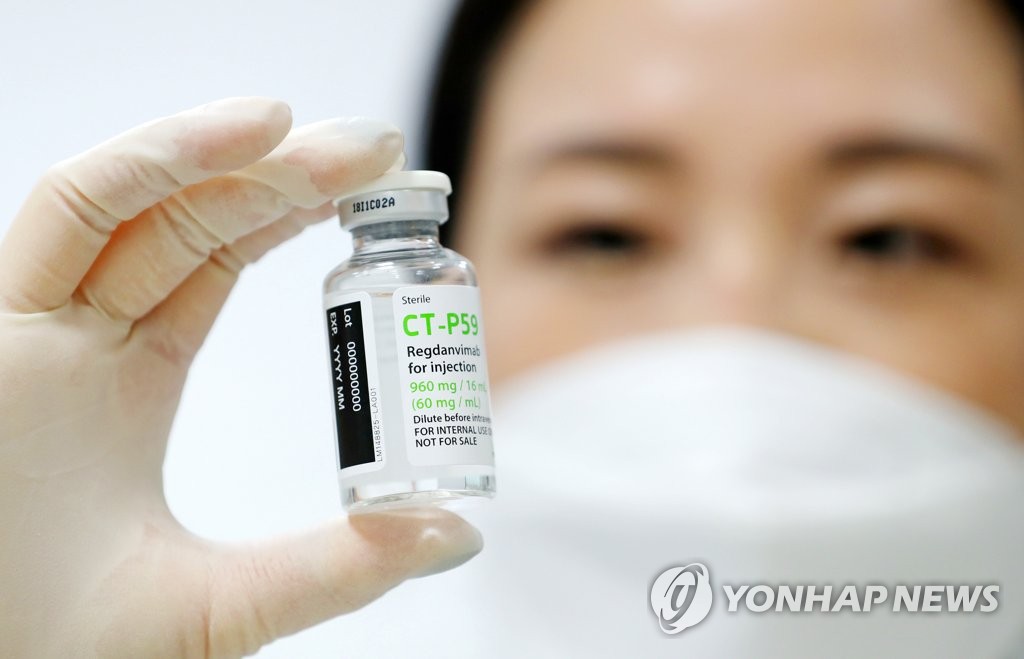
(Seoul = Yonhap News) Celltrion’s self-developed novel coronavirus infection (Corona 19) antibody treatment “Recyronaju” (ingredient name legdanvimab code name CT-P59) shortens the recovery period of patients as a result of phase 2 clinical trials. , It has been shown to lower the incidence of severe cases. Celltrion released data on phase 2 clinical trials conducted on 327 patients with mild to moderate corona19 through a public announcement on the 13th. The photo shows the cure that was released to the media on December 22 last year. 2021.1.13 [연합뉴스 자료사진] [email protected]
(Seoul = Yonhap News) Reporter Ji-Heon Lee = Celltrion, announcing the results of phase 2 clinical trials of an antibody treatment for novel coronavirus infection (Corona 19)[068270]It fell sharply on the 14th.
On this day, Celltrion closed the deal at 352,500 won, down 29,000 won (-7.6%) from the previous day.
Celltrion Pharmaceutical in charge of pharmaceutical production and global sales[068760](-9.84%) and Celltrion Healthcare[091990](-8.19%) also plunged.
Earlier, Celltrion announced the results of phase 2 clinical trials related to the Corona 19 antibody treatment’Recyronaju’ (ingredient name Regdanvimab code name CT-P59) after the closing of the market the day before.
Celltrion found that Rekironaju reduced the incidence of severe patients requiring inpatient treatment by 54% in all patients and 68% in moderately ill patients over 50 years old. He added that the recovery period was shortened by more than 3 days on average.
Heo Hye-min, a researcher at Kiwoom Securities, said, “Especially in the elderly and high-risk groups, the hospitalization period has been shortened.”
Lee Myung-sun, researcher at Shinyoung Securities, said, “It is difficult to directly compare the efficacy evaluation of clinical results with the antibody treatments of Eli Lilly and Regenenon, which were approved for emergency use by the US Food and Drug Administration (FDA) in November last year, but in terms of safety, Celltrion “I think Naju is excellent,” he said. “However, it is difficult to reflect the hasty results in that there are not many confirmed cases in Korea, and approval is ahead.”
Lee Dong-gun, a researcher at Shinhan Investment Corp., predicted, “Considering the case of urgent use approval of an antibody treatment, it is expected that emergency use approval can be obtained as early as February.”
Unauthorized reproduction-redistribution prohibited>
2021/01/14 15:50 sent