Scheduled for the first expert verification on the 17th
(Seoul = Yonhap News) Reporter Jandi Kim = The drug authorities have been thorough about Celltrion’s new coronavirus infection (Corona 19) antibody treatment “Recyronaju” (ingredient name Legdanvimab codename CT-P59), which has received a conditional permit application. I went to the verification.
The Ministry of Food and Drug Safety announced on the 14th that it will hold a meeting of the’Corona 19 Treatment and Vaccine Safety and Effectiveness Verification Advisory Group’ (hereinafter referred to as Verification Advisory Group) meeting from experts to verify and advise on the results of clinical trials in Rekirona. The results will be released on the 18th.
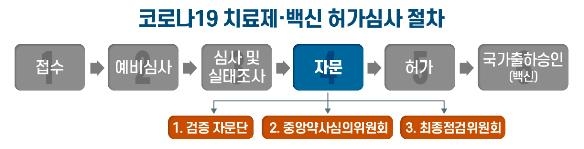
The Ministry of Food and Drug Safety has prepared a procedure for consulting external experts in a’triple’ manner to objectively and transparently review the approval of COVID-19 treatments and vaccines.
Consultation consists of the verification advisory group, the central pharmacist review committee (central pharmaceutical review committee), and the final inspection committee.
The verification advisory meeting is the first step in the expert advisory process.
![[식품의약품안전처 제공. 재판매 및 DB 금지]](https://i0.wp.com/img7.yna.co.kr/etc/inner/KR/2021/01/14/AKR20210114053300017_01_i_P4.jpg?w=560&ssl=1)
[식품의약품안전처 제공. 재판매 및 DB 금지]
The verification advisory group is a procedure for the Ministry of Food and Drug Safety to collect advisory opinions on areas such as clinical, non-clinical, and quality from various experts prior to consulting with the Central Pharmacy. 30 experts focused on infectious medicine will participate.
At a meeting of the verification advisory group for Rekirona, the results of clinical trials are appropriate to acknowledge the therapeutic effect of this drug.
Celltrion announced that the administration of Rekironaju to mild and moderate Corona 19 patients the previous day reduced the recovery period by more than 3 days and reduced the incidence of severe patients by 54%.
After the verification advisory group meeting, opinions are sought from the Central Pharmaceutical Affairs Advisory Committee, an advisory body of the Ministry of Food and Drug Safety. The advisory committee for the Central Pharmacopoeia is composed of about 15 members, including standing members of the Biological Drug Subcommittee. This section deals with the issues discussed by the verification advisory group and its clinical usefulness.
After that, the Ministry of Food and Drug Safety holds a final inspection committee in which 10 internal and external experts participate in joint participation to determine the final permission. A final review will be conducted based on the results of the verification advisory group and the central pharmacy review committee.
Unauthorized reproduction-redistribution prohibited>
2021/01/14 10:02 sent