 |
| The Minister of Food and Drug Safety, Kim Kang-rip, is giving a briefing on the results of the AstraZeneca Corona 19 Vaccine Final Inspection Committee meeting at the briefing room of the Ministry of Food and Drug Safety in Osong-eup, Cheongju-si, Chungbuk on the 10th. From left, Yu-hwan, Chairman of the Central Pharmacy Review Committee, Kim Sang-bong, Director of the Bio-Pharmaceutical Bureau, Ministry of Food and Drug Safety, and Kim Kang-lip, Director. /News1 © News1 Reporter Jang Soo-young |
AstraZeneca Corona 19 vaccine was given a warning that it should be carefully administered to people 65 years of age or older, and the final marketing approval was granted, and the Korea Centers for Disease Control and Prevention, which planned to inoculate the vaccine to the elderly from the end of this month, was in a rather complicated situation.
On the 10th, the Ministry of Food and Drug Safety Final Inspection Committee determined that the current clinical data, which lacked the number of elderly subjects, were insufficient to confirm the preventive effect for them. However, as the safety standards were passed and the effect was confirmed in all clinical trials over 18 years of age, the vaccination was allowed once. Instead, it was decided to write the phrase’the use of the product should be carefully decided for the elderly over the age of 65′ so that doctors can judge the precautions for using the product.
In fact, the Ministry of Food and Drug Safety has opened the way to vaccination for the elderly. However, as the unpleasant condition of’careful administration’ arises, the deliberation of the’Expert Advisory Meeting’ and the’Vaccination Specialized Committee’ of the Disease Administration, which will be held in the future, is predicted to change the priority order of vaccinations or proceed as planned. The Agency for Disease Control and Prevention usually finalizes the vaccination plan through these two deliberation steps before the new vaccine is used domestically.
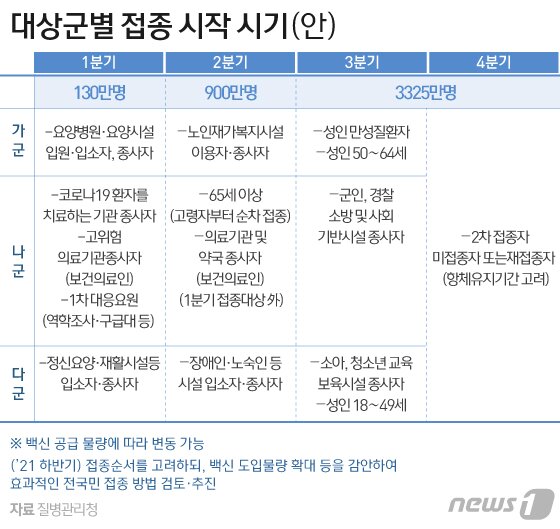
According to the Korea Centers for Disease Control and Prevention on the 11th, the AstraZeneca vaccine will be administered sequentially to 750,000 people (1.5 million doses) from the 26th to residents and workers in nursing hospital facilities.
This is a plan set up by the government ahead of time and has not changed yet. However, the deliberation results of the’Expert Advisory Meeting’ and the’Vaccination Expert Committee’ can act as variables. The Agency for Disease Control and Prevention plans to finalize the vaccination plan after deliberation by the Vaccination Specialist Committee by the 19th.
The Ministry of Food and Drug Safety requested AstraZeneca to submit US clinical data, including a significant number of the elderly, by the end of April to completely eliminate the condition of’careful administration’ for the elderly. The final approval of the Ministry of Food and Drug Safety was made under these conditions.
At a briefing on the 10th, the head of the Food and Drug Administration said, “We requested an interim report and a final report on clinical trials currently in progress in the United States.” “I have imposed a condition to submit it.”
Therefore, the cautious administration of the elderly is bound to continue for a period of two months. During this period, administration of patients in nursing hospital facilities with the elderly (from the end of February) and patients aged 65 (from the second quarter) is planned. It is a situation that cannot rule out the possibility of voices that cannot be vaccinated among the medical staff or those who receive the vaccine.
A government official said, “I know that the debate about the administration of people over 65 years of age among experts at the Central Pharmacy Center has been prolonged.” It seems to be,” he said.
 |
| © News1 Designer Eunhyun Lee |
On the other hand, there is a view that the vaccination will proceed as planned, as the approval authority, the Food and Drug Administration, allowed the prescription. Above all, as expected by the government, even if the Pfizer vaccine is introduced in February, the only vaccine that can be vaccinated by the elderly is the AstraZeneca vaccine, and it is difficult to delay the vaccination time any more.
Unlike AstraZeneca vaccine, Pfizer vaccine is a vaccine manufactured based on genetic mRNA, and due to its nature, it must be vaccinated at a vaccination center that can be stored frozen. In particular, the elderly in nursing homes are generally difficult to move, so it is difficult to move to this center, so the government has planned an inoculation vaccine for them with AstraZeneca products.
Another government official said, “As the overall clinical effect of the AstraZeneca vaccine has been confirmed, the Ministry of Food and Drug Safety has granted permission for those over 18 years of age. There is doubt about the effect of the elderly, but there is no physical evidence, so they will wait for related data.” It is also difficult to delay the vaccination time, so there is a possibility that the vaccination will proceed as planned.”
The Agency for Disease Control and Prevention plans to inoculate the AstraZeneca vaccine for residents and workers in nursing hospitals or facilities from the 26th.
Hong Jeong-ik, head of the vaccination management team at the Korean Disease Service, said in an online back briefing of the access reporters on the 10th, “AstraZeneca vaccine will be supplied to the distribution center on the 24th, and if you look at the subdivision, packaging, and delivery schedules to medical institutions, it will be about 26th. “There is room for fluctuations that will be pulled by a day if we hurry more,” he said. The AstraZeneca vaccine to be used this time is produced by a Korean company, SK Bioscience.
