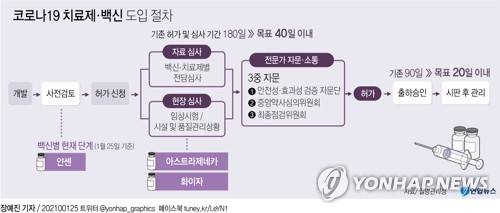
AstraZeneca’s new coronavirus infection (Corona 19) vaccine, which is about to be introduced in Korea, is going through the first expert consultation process by the authorities this weekend.
![Corona 19 treatment/vaccine introduction procedure [연합뉴스]](https://i0.wp.com/news.google.com/edb/nimages/2021/01/2021013009261678110.jpg?w=560&ssl=1)
Corona 19 treatment/vaccine introduction procedure [연합뉴스]
According to the pharmaceutical industry on the 30th, the Ministry of Food and Drug Safety held a’Corona 19 Vaccine Safety and Effectiveness Verification Advisory Group’ (hereinafter referred to as the Verification Advisory Group) meeting on the afternoon of the 31st with external experts regarding clinical trial data for AstraZeneca Corona 19 Vaccine (AZD1222). Open. The results will be released on February 1.
The Ministry of Food and Drug Safety has prepared a procedure for consulting external experts in’triple’ for objective and transparent approval review for COVID-19 treatments and vaccines. Consultation consists of the verification advisory group, the central pharmacist review committee (central pharmaceutical review), and the final inspection committee.
At the verification advisory meeting, clinical experts who focus on infectious medicine will participate to examine the safety and effectiveness of AstraZeneca Corona 19 vaccine, clinical significance, and the appropriateness of target patients.
AstraZeneca applied for an item permission for the Corona 19 vaccine with the Food and Drug Administration on January 4 this year. Among the corona 19 vaccines to be introduced in Korea, it is the first product to apply for product permission.
![AstraZeneca Corona 19 Vaccine [AFP=연합뉴스 자료사진]](https://i0.wp.com/news.google.com/edb/nimages/2021/01/2021013009261702830.jpg?w=560&ssl=1)
AstraZeneca Corona 19 Vaccine [AFP=연합뉴스 자료사진]
This product is a vaccine that is administered twice in total, and its storage conditions are 2-8℃, so it can be distributed under the existing system without the need to establish a separate cryogenic system.
It is expected that supply and demand will be smooth as it has signed a consignment production contract with SK Bioscience, a domestic vaccine company. Korea has signed a vaccine purchase contract with AstraZeneca for 10 million people.
However, in the case of the AstraZeneca vaccine, it is known that the authorities will thoroughly verify this part as a controversy about the efficacy of the vaccine for the elderly over 65 has risen.
The Ministry of Food and Drug Safety is comparing and reviewing the vaccination group and the placebo group to see if there is any safety information that requires special attention from the elderly over 65 years of age.
An official from the Ministry of Food and Drug Safety said, “We will thoroughly verify the safety and effectiveness through a triple expert consultation procedure starting on the 31st,” and said, “There is no specific decision on the scope of the permit, since expert consultation has not yet started.”
![Domestic Corona 19 vaccine supply schedule [연합뉴스]](https://i0.wp.com/news.google.com/edb/nimages/2021/01/2021013009261729242.jpg?w=560&ssl=1)
Domestic Corona 19 vaccine supply schedule [연합뉴스]
On the 29th (local time), the European Union (EU) executive committee approved conditional sale of AstraZeneca’s COVID-19 vaccine. Following the vaccine developed by Pfizer and Modena, it is the third COVID-19 vaccine approved by the EU for conditional use.
The EU’s decision came a few hours after the European Medicines Agency (EMA) recommended conditional marketing approval for the vaccine to all age groups over 18 years of age.
EMA said that the AstraZeneca vaccine was found to be safe and effective in preventing COVID-19 in people over the age of 18 when the results of clinical trials conducted in the UK, Brazil and South Africa were combined.
yunhap news