■Advice result of the Central Pharmacy Review Committee
“Statistical utility is not revealed…more discussion”
Controversy spreads over rejection of Swiss line approval
Nursing hospital workers instead of the elderly
‘Pfizer Vaccine’ may be given first
 viewer
viewer
As the Ministry of Food and Drug Safety and the Central Pharmacy Review Committee reserve the judgment on whether to inoculate the AstraZeneca vaccine for the elderly aged 65 or older, there is concern about disruption to the vaccine vaccination plan for the novel coronavirus infection (Corona 19).
Yoo-hwan, chairman of the Ministry of Food and Drug Safety’s Central Pharmaceutical Affairs Commission, recommended that the vaccination decision for the elderly 65 years or older be discussed at the Vaccination Committee on the 5th was “because the effectiveness at the level to be statistically verified has not been verified.” “To further analyze the effectiveness of the elderly. It is recommended that additional US clinical results be submitted in the future.” Among the participants in the AstraZeneca vaccine clinical trial, only 9.7% of all participants over 65 years of age are criticized around the world.
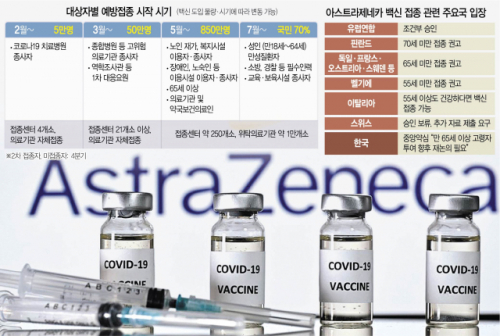
The announcement of the Central Pharmacopoeia is a decision to step back from the previous primary vaccine verification advisory group. The advisory group commented that “the administration to the elderly cannot be excluded just because the number of elderly people among the participants is small.” Some experts said, “It is desirable to reflect the vaccination for elderly people in the permission matters after checking the additional results.” During a total of three consultations, the vaccination for the elderly was braked in the second round. The Ministry of Food and Drug Safety will hold a final review committee, a final advisory body, to decide whether to approve AstraZeneca vaccine, and the Korea Centers for Disease Control and Prevention (KCDC) will open a vaccination committee to discuss specific vaccination plans including whether or not to vaccination for the elderly.
The effectiveness of the AstraZeneca vaccine for the elderly is also controversial in Europe. According to the recommendation of the European Medicines Agency (EMA), the European Union (EU) Commission has allowed AstraZeneca vaccine to be vaccinated for all age groups over the age of 18, but some countries, such as Germany, France, Austria and Sweden, are under 65. This vaccine was recommended for subjects. Switzerland has withheld approval of the AstraZeneca vaccine altogether. Italy recommended that people under the age of 55 should use the vaccine first, but recently revised its opinion that even over the age of 55 can get the vaccine if they are healthy.
As conclusions on vaccination for the elderly have been withheld, there are concerns that the previously planned vaccination schedule may also be disrupted. AstraZeneca is scheduled to release the results of clinical trials for the elderly in April, but if the vaccination decision is postponed until then, the vaccine is not available for the elderly. The government has made plans to inoculate about 766,900 people, including patients and workers admitted to 5,692 nursing hospitals, nursing homes for the elderly, and 5,692 mental care and rehabilitation facilities by the first quarter of this year. AstraZeneca vaccine can be stored at 2 to 8 degrees, so it is easy to inoculate directly to a nursing hospital, so it is highly likely to be inoculated to the elderly. However, if AstraZeneca vaccination is restricted, it is difficult to visit 250 vaccination centers nationwide to get Pfizer vaccination, which is practically difficult due to the nature of elderly patients. Eun-mi Chun, a professor of infectious medicine at Ewha Womans University Mokdong Hospital, said, “Because the vaccines coming in the first quarter are limited, the targets of vaccination may change. We may hit the nursing hospital workers first, and then advance the vaccination of institutional workers such as the police and firefighters, which are the next priority. . Jeong Jae-hoon, a professor of preventive medicine at Gachon University Medical School, said, “It may be a little late, but I don’t think it will be too late. In the UK, we are vaccinating people over 65 years old, and if the data comes out, the controversy about the effectiveness will be reduced. Explained.
 viewer
viewer
/ Reporter Kim Seong-tae [email protected]
< 저작권자 ⓒ 서울경제, 무단 전재 및 재배포 금지 >