 |
| © News1 Designer Eunhyun Lee |
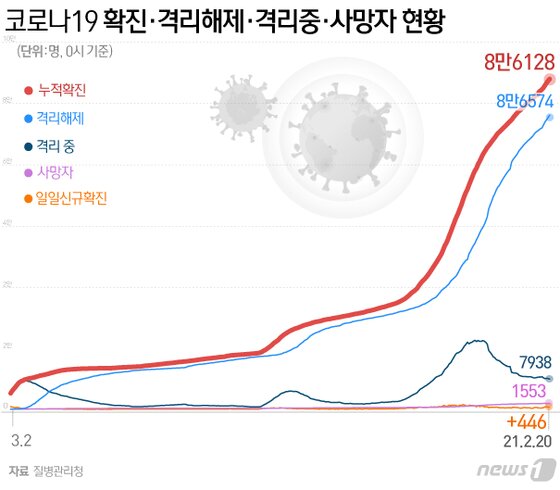
From the 26th, the vaccine vaccination against Corona 19 will be in full swing in Korea. To this end, a list of 340,000 people who wished to receive vaccination among the first-ranked vaccination subjects was confirmed. However, 6.2% of the first-ranked subjects refused the vaccination. In particular, 5.4% of the first-ranked medical staff refused to receive vaccination.
This suggests that there are many potential concerns about the safety and effectiveness of the vaccine due to controversy over vaccination of the AstraZeneca vaccine and side effects reported overseas. Accordingly, many point out that it is necessary to prepare a device to relieve anxiety in order to actively participate in vaccinations in the future.
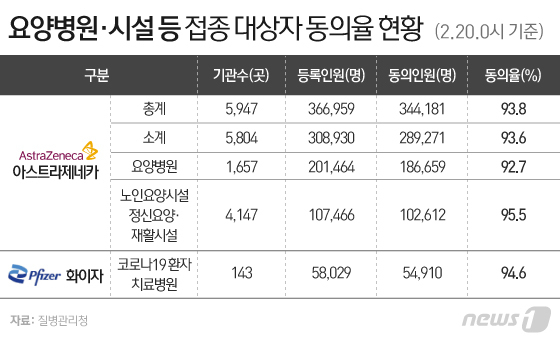
◇ 6.2% of the 36,6959 people who were eligible for the first-rank vaccination refused
According to the Centers for Disease Control and Prevention of the Korea Centers for Disease Control and Prevention (KCDC) on the 21st, it was confirmed that as of 0 o’clock on the 20th, 340,4181, 93.8% of the 36,6959 of the first-ranked vaccinated patients, agreed to vaccination. Conversely, 6.2% (27,782) of the vaccination targets refused to be vaccinated.
Workers in nursing hospitals and facilities and residents will receive the AstraZeneca (AZ) vaccine, and pfizer vaccines will be given to workers such as medical personnel working at the COVID-19 treatment hospital.
The vaccination rejection rates of the two vaccines were 6.4% and 5.4%, respectively, for AstraZeneca and Pfizer. Regardless of the vaccine product, the rate of vaccination targets 5-6% shows rejection of vaccination itself. In particular, it is of concern that the rate of medical personnel refusing vaccination is in the 5% range.
The corona 19 vaccine, which is administered for the first time in Korea, is a product of AstraZeneca (AZ), a British pharmaceutical company. Due to the lack of clinical data on the elderly over 65 years of age, controversy about the efficacy arose, the rate of consent for vaccination was relatively low compared to the Pfizer vaccine.
AstraZeneca vaccine was administered by 92.7% of the 20,1464 people who were vaccinated at 1657 nursing hospitals. Inpatient consent rate was 90.0%, and worker consent rate was 93.9%. In addition, 95.5% of the 1,08466 people eligible for 4147 elderly care facilities, mental care, and rehabilitation facilities agreed. Pfizer vaccination targets 94.6% of 5,829 workers in 143 corona19 patients treatment hospitals nationwide agreed to receive the vaccine.
Lee Sang-won, director of epidemiological investigation and analysis, said at a briefing on the 20th, “From the last 8th to the Corona 19 treatment hospital, from the 10th to the nursing hospital, nursing facility, mental nursing facility, and rehabilitation facility, register the vaccination target and confirm the consent to vaccination. The same rate of consent came out.”
 |
| A new coronavirus infection (Corona 19) vaccine distribution The second pan-government integrated simulation training was held on the afternoon of the 19th at the Gwanak-gu Health Center in Seoul, where an official is putting the AstraZeneca vaccine into a dedicated refrigerator./News1 © News1 Reporter Lee Donghae |
◇ AZ vaccine continues controversy over administration of elderly people… The United States is hesitant to immunize one-third of the population
In particular, AstraZeneca vaccine will be administered to the first inoculation in Korea. However, the vaccine was only allowed to be vaccinated under the age of 65 due to lack of clinical data on elderly people over 65 years old. Vaccinations over the age of 65 will be confirmed after the end of March when additional clinical data are available through deliberation by the Vaccination Specialist Committee.
Given this situation, voices of worries about vaccination do not fade in Korea. The situation in the United States, which has suffered from a massive infection, is even worse. Researchers at the University of California’s Davis campus (UC Davis) recently revealed their findings that more than one-third of Americans are hesitant to get the COVID-19 vaccine. The results of this study were published in the international journal’Vaccine’ earlier this month.
The four reasons Americans are reluctant to be vaccinated against the COVID-19 vaccine are: △concerns about side effects of the vaccine △worries about allergic reactions after vaccination △doubt about the effectiveness of the vaccine △preferred immunity generated naturally after infection.
Domestic medical experts also have different opinions. Although the vaccine itself is not a problem, there are voices that sufficient clinical data must be secured in consideration of safety, effectiveness and public acceptance.
Professor Nam Jae-hwan of the Department of Biomedical Science at Catholic University emphasized, “All vaccines introduced in Korea other than the AstraZeneca vaccine show a high antibody production rate.” .
Eun-mi Cheon, a professor of respiratory internal medicine at Ewha Womans University Mokdong Hospital, said, “First of all, clinical data over 65 years of age are currently lacking, but since data is available from abroad at the end of March, the vaccination is not very late even if you judge this result.” AstraZeneca vaccine is effective only when a total of two doses are given.
 |
| © News1 Designer Eunhyun Lee |