 |
| © News1 Designer Kim Il-hwan |
The quarantine authorities will announce a vaccination plan for a novel coronavirus infection (Corona 19) vaccine on the 28th. As for the Corona 19 vaccine, most of the contents have been kept’private’, so it is expected to be a’comprehensive announcement’.
The announcement of the vaccination plan is expected to cover the distribution/storage plan of the vaccine, the vaccination method, etc., from the priority vaccination targets with the highest interest.
In addition, as there was controversy such as reporting a death case during the last flu influenza vaccination process, plans for post-vaccination management will also be presented.
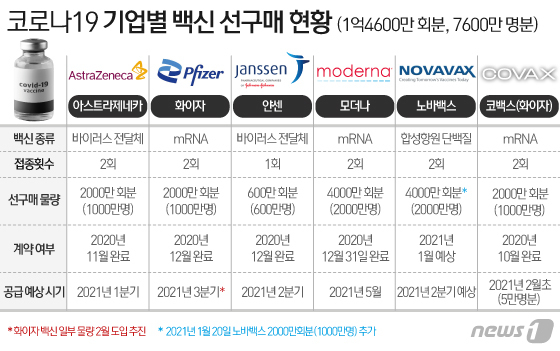
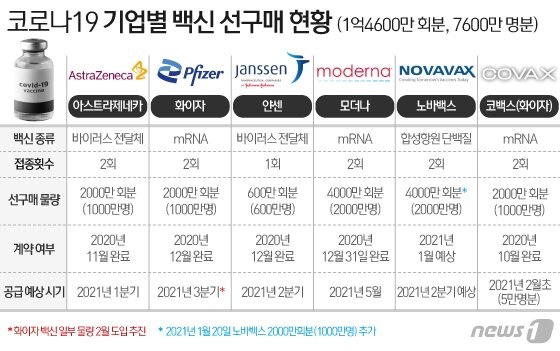
◇ Preparation for introduction of 76 million people… Pfizer vaccine introduced in early February through COVAX
Ko Jae-young, head of the crisis communication team at the Central Defense Response Headquarters, said at the regular briefing for Corona 19 on the 23rd, “A briefing on the implementation of the Corona 19 vaccination will be held on Thursday the 28th.”
Currently, the Korean government is promoting the introduction of vaccines for 76 million people. Through individual negotiations with global pharmaceutical companies, we have completed a pre-purchase agreement for 46 million people, including △10 million AstraZeneca △10 million Pfizer △ 20 million Moder or △ 6 million Janssen, and COVAX facility, a vaccine purchasing alliance. ) Also has applied for 10 million vaccines. In addition, the government plans to purchase an additional 20 million doses of NovaVax vaccine.
Of these, the first vaccine to be introduced in Korea is expected to be the COVAX vaccine. According to the government, Kovacs is currently conducting a demand survey for applicant countries.
When this process is completed, the introduction of the COVAX vaccine is expected to be in early February. This is earlier than the end of February of the AstraZeneca vaccine, which was initially expected to be introduced the earliest.
The initial volume of COVAX vaccine is estimated at 50,000 Pfizer vaccines. Pfizer vaccine is still subject to review by the Ministry of Food and Drug Safety, but the announcement on the 28th is expected to include an inoculation plan for the amount.
◇Priority vaccination target medical staff strong… ‘Sinjoong’ of the elderly in overseas side effects
The most interesting part is who gets the Corona 19 vaccine first. Until now, the first vaccination by medical staff is the most effective.
The priority vaccination targets set by the government are △ medical institution workers △ group facility residents and workers △ 65 years old or older △ adults with chronic illnesses △ children and adolescents education and childcare facilities workers and employees △ Corona 19 first responders △ Adults aged 50 to 64 △ Police, firefighting officials, military personnel △ Correctional facilities and treatment detention center prisoners and staff.
Initially, senior citizens living in nursing homes were considered for priority vaccination, but recently, overseas side effects of the Pfizer vaccine were reported, so it seems that they turned to medical staff.
Recently, 33 deaths have been reported in Norway following Pfizer vaccination. All of them are known to have suffered from chronic diseases at the age of 75 or older.
◇Pfizer vaccine screening plan may also be included… SK Bioscience, Cold Chain Management
It is also expected that Pfizer vaccine review and distribution and storage plans will be mentioned.
In order for Pfizer vaccine to enter Korea in early February, it must first obtain an item permission from the Ministry of Food and Drug Safety. The Ministry of Food and Drug Safety has shortened the review period to less than 40 days from application to approval, and it is expected that the application for Pfizer vaccine approval will be made soon considering the timing of introduction.
As Pfizer’s vaccine was first approved in the US and Europe, the domestic approval review period may be earlier than expected. However, since it was introduced in early February, it is necessary to go through the procedure for national shipment approval (national inspection) by the Ministry of Food and Drug Safety. The national inspection review usually takes 2 to 3 months, but the Ministry of Food and Drug Safety has set up a professional team for this Corona 19 vaccine and treatment, and has made plans to complete it within 20 days. Therefore, the timing of physical vaccination is expected to be after mid-February as early as possible.
In addition, the government has selected SK Bioscience as a company dedicated to establishing a distribution management system for vaccines. SK Bioscience, which has already decided to take over the production of AstraZeneca and NovaVax vaccines, has been selected as a storage and distribution agency to transport the vaccine to the inoculation center.
SK Bioscience plans to operate a system that can monitor temperature maintenance and delivery routes in real time during vaccine transportation by establishing a customized cold chain (low temperature distribution) management system for each vaccine. It is expected that this will be included in the comprehensive plan on the 28th.
The government is preparing a separate vaccination center as Pfizer vaccine and Modena vaccine are mRNA-based vaccines and must be stored and transported in a cryogenic environment (minus 20-70 degrees Celsius).
The Ministry of Public Administration and Security is planning to operate 250 vaccination centers. The standard was set to select one or more per city, county, district, and three places where the population exceeds 500,000. 150 sites were selected as of 6 pm on the 20th, and the remaining 100 sites are also scheduled to be selected early next week.
◇ Adverse reaction monitoring through inoculation system… “Around November, 70% of the people are expected to be immunized”
It is also planning to introduce a surveillance plan for side effects after the COVID-19 vaccination.
Earlier, the quarantine authorities had suffered from measles during the 2020-2021 influenza vaccination process. Following cold exposure to flu vaccines and white particles, 108 deaths were reported. As a result of the epidemiological investigation and deliberation of the damage investigation group, the causality between death and vaccination was not recognized, but public anxiety increased.
First of all, various side effects have been reported overseas, where the Corona 19 vaccination was started.
Through the vaccination system, the government plans to prevent the inoculation from being focused on specific vaccination centers or medical institutions, and provide guidance on the second vaccination management, issuance of vaccination certificates, and monitoring of adverse reactions.
In addition to the Covax (Pfizer) and AstraZeneca vaccines, which are expected to be introduced first, △Modena vaccine in May △Jansen 2Q △Pfizer 3Q (excluding Covax supply), and NovaVax vaccine is also expected to be introduced in 2Q. Is expected. It is also expected to announce a sequential introduction plan for this. Through this, it is a policy to form a collective immune system in which more than 70% of the nation has antibodies.
Minister of Health and Welfare Kwon Deok-cheol said at a press conference on the 21st, “The vaccination is completed in September, and accordingly, around November, 70% of the people are expected to become group immunity.”