 |
| © News1 Designer Choi Soo-ah |
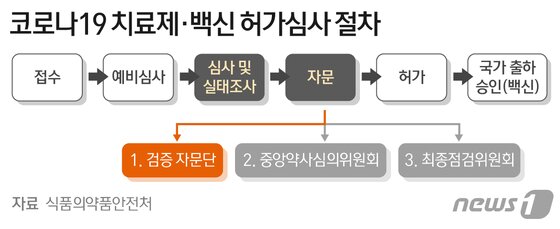
Celltrion’s’Corona 19′ antibody treatment’Rekironaju (project name: CT-P59)’ was conditionally approved by the Ministry of Food and Drug Safety Verification Advisory Group, which verified the safety and effectiveness of the results of phase 2 clinical trials. And recommended separate phase 3 clinical trial).
As a result, Rekkironaju has crossed the 9th ridge and the possibility of final product approval has increased. In the future, the Ministry of Food and Drug Safety will synthesize these results and receive advice from the Central Pharmacy Review Committee, the legal advisory body of the Ministry of Food and Drug Safety, and finally decide whether to approve the product.
Kim Sang-bong, head of the Food and Drug Administration’s Bio-Pharmaceutical Bureau, announced the results on the 18th through a briefing on the status of the Corona 19 treatment and vaccine approval status. Director Kim said, “The verification advisory group proposed product approval for Rekirona on the premise of conducting phase 3 clinical trials.”
The verification advisory group meeting is a procedure in which the Ministry of Food and Drug Safety collects advisory opinions on areas such as clinical, non-clinical, and quality from various experts before consulting the Central Pharmacy.
At the advisory group meeting held on the 17th, the clinical results of Rekirona’s’Clinical Effectiveness Measurement Index’ and’Measurement Index of the Principles of the Drug’ were asked whether it is appropriate to acknowledge the therapeutic effect of this drug. The meeting was attended by eight external experts in the field of clinical trials, including infectious medicine specialists, virology specialists, and clinical statistics specialists, as well as four persons including the general review team and clinical evaluation team of the’Corona 19 treatment approval review team’ inside the Ministry of Food and Drug Safety.
As a result of consulting on the indicators for measuring clinical effectiveness, it was confirmed that Rekyrona took 5.34 days to recover from the symptoms of Corona 19 and shortened it by 3.43 days from 8.77 days for patients taking placebo. The verification advisory group determined that this was statistically significant.
This indicator evaluates how quickly the’rekironaju’ group and the placebo group recover from the 7 symptoms of Corona 19 (fever, cough, difficulty breathing, sore throat, systemic pain (muscle pain), fatigue, and headache).
In the indicators for measuring the principle of the drug’s operation, the decrease in the time between the positive to negative conversion of Rekirona strain as a result of the virus test was not statistically significant, but it was suggested that there was a tendency to decrease the virus concentration in the body after administration.
In this clinical trial, the rate of patients who are hospitalized or need oxygen therapy due to Corona 19 has decreased by administering Rekirona in the’inpatient rate of hospitalization/oxygen therapy’, which is an auxiliary method to confirm the effect.
From the time of administration to the point of time 28 days elapsed, the safety of Rekirona was passed. Hypertriglyceridemia and hypercalcemia that occurred after receiving this drug were already confirmed in phase 1 clinical trials and were predictable abnormal cases.
In general, minor or moderate adverse events occurred, but compared with the Rekirona injection group and the placebo group, the ratio was similar and there were no serious life-threatening adverse events.
Director Kim Sang-Bong said, “The Ministry of Food and Drug Safety conducts review of the submitted data, including the opinions and recommendations of the verification advisory group, and some of the quality data that are still remaining, and synthesizes the results to ensure safety, effectiveness, and matters to be considered in the case of approval. “I plan to get advice about it.”
![[속보]Ministry of Food and Drug Safety Verification Advisory Group “Celltrion Corona 19 Antibody Drug, Conditional Permission Recommendation” [속보]Ministry of Food and Drug Safety Verification Advisory Group “Celltrion Corona 19 Antibody Drug, Conditional Permission Recommendation”](https://image.news1.kr/system/photos/2021/1/18/4579749/article.jpg)