 |
| © AFP=News1 |
AstraZeneca (AZ) vaccine had the highest incidence of adverse events for the novel coronavirus infection (Corona 19) vaccine reported in France, followed by Pfizer vaccine and Modena vaccine. However, in terms of ratio, all three vaccines were less than 1%.
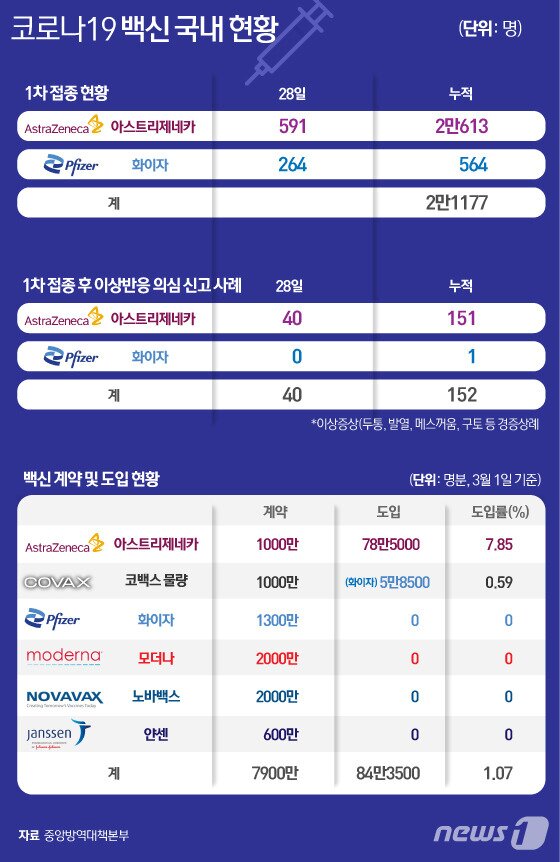
Most of the side effects of the AstraZeneca vaccine, which has been vaccinated the most in Korea so far, are mostly mild, and most of the symptoms disappeared within a day.
◇France side effects incidence rate AZ, Pfizer, Modena, in order… All less than 1%’mild’
According to the industry on the 2nd, the French Ministry of Food and Drug Safety (ANSM) summarized and disclosed side effects for each corona19 vaccine identified in their country on the 26th of last month (local time).
The number of side effects by vaccine varied with △ 5331 Pfizer cases △ 971 AstraZeneca cases △ 148 cases of Moder or △. However, the incidence of side effects compared to those inoculated was less than 1%, such as △Asrazeneca 0.55%, △Pfizer 0.16% △Moder or 0.11%, and most of the symptoms were mild.
In the case of the Pfizer vaccine’Cominati’, out of the 3.3296 cases of vaccinations conducted from December 27 last year to the 18th of last month, the reported side effects were 5,331 cases. There were 169 reported deaths after vaccination, but there is no known association between the deaths and the vaccine.
The most common side effects of Pfizer vaccine were fever, muscle pain, and other symptoms similar to those of flu vaccination. The symptoms were more likely to appear after the second vaccination than the first vaccination. Some serious cases have also been reported. There were 75 cases related to hypertension such as dizziness, 56 cases related to cardiovascular disease, and 10 cases of facial paralysis.
Of the side effects that occurred after vaccination with the AstraZeneca vaccine, 93% were pain, inflammation, digestive and muscle pain at the injection site, which were similar to those of the flu vaccination. 72% of them disappeared within 24 hours.
According to the Financial Times on the 27th (local time), the AstraZeneca vaccine distributed in France is about 170,000 doses, which is about 16% of about 1.1 million doses (one dose is one dose). The incidence rate of side effects is about 0.55%.
French authorities explained that these side effects are evidence that the vaccine triggers an immune response in the body. However, 4 cases of some serious side effects were also reported. After vaccination, 1 stroke and 2 taste loss were reported. One person died after vaccination, but the death does not appear to be associated with the vaccine yet.
Among the people vaccinated with’mRNA-1273′ in Modena in the United States, there were 148 side effects. These cases mainly complained of problems with skin, muscles, and digestion, and four of them had cardiovascular-related problems and had serious symptoms.
One of the people who received the Modena vaccine died, but no association with the vaccine was found. Since January 22nd, in France, 129510 people have been vaccinated at least once.
◇ AZ Corona 19 Vaccine Even in Germany, etc. over 65
The AstraZeneca vaccine has been restricted in some countries because of insufficient data from clinical trials in the elderly over the age of 65. Korea is also in a state of delaying vaccinations for elderly people 65 years of age or older until clinical data are confirmed.
However, a recent study in the UK showed that the AZ vaccine significantly lowered the risk of hospitalization for vaccinators. A study conducted in the Scottish region found that the AZ vaccine reduced the risk of hospitalization for vaccinators by 94%, and significantly reduced the risk of hospitalization for seniors aged 65 and over.
In response to the news, the German government, which had withheld the AZ vaccination for people 65 years of age or older, changed its position to allow the vaccination again. The Canadian government, which recently licensed the vaccine, is also planning to start vaccinating the vaccine even over the age of 65. The French government is also planning to start vaccinating Corona 19 for people aged 65 to 74 from April.
Korea’s quarantine authorities are also in the position to confirm the effectiveness of the vaccine for those over 65 years of age.
“As a result of comparing the (AstraZeneca vaccine) vaccination in Scotland, it was announced that the effect of preventing serious diseases such as hospitalization rate (in the elderly) is quite high,” said Eun-kyung Chung, head of the Central Defense Response Headquarters of the Centers for Disease Control and Prevention. “We plan to collect data from time to time and carefully re-discuss and review whether AstraZeneca (elderly vaccines) will be used,” he said.
 |
| © News1 Designer Il-Hwan Kim |
