Celltrion, a pharmaceutical company that develops the first COVID-19 treatment in Korea, is speeding up clinical trials to develop a’cocktail antibody’ that will fight against various mutant viruses, following Rekirona, which is currently conditionally licensed by health authorities.
Celltrion revealed its plans to develop future treatments at an emergency online press conference held on the 18th. Celltrion Group Honorary Chairman Seo Jeong-jin, Celltrion Research & Development Headquarters Kwon Ki-seong, and Celltrion Clinical Planning Chief Kim Seong-hyun attended the conference.
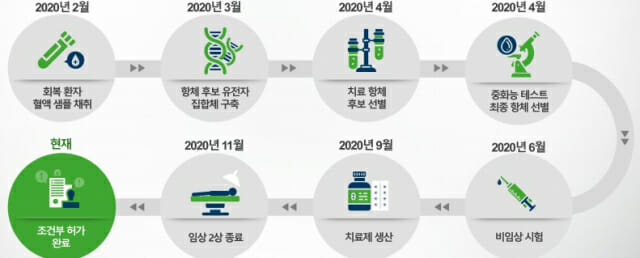
Currently, in the case of Rekkirona supplied to domestic medical institutions with conditional approval from the Ministry of Food and Drug Safety, the results of phase 3 clinical trials must be submitted within the year. Currently, it has been 1 month since the administration of Rekirona to patients for phase 3 has been started, and it has been administered to 150 patients. It is expected that it will take 3 months to complete administration to 1,172 patients subject to phase 3 and about 5 months to analyze the data. It is also expected to secure effectiveness results for mild patients.
Regarding the expected production volume of Rekirona, the honorary chairman said, “Currently, 100,000 people are made for domestic use. I will see and decide.”
Upon completion of Rekirona Phase 3, vaccine dosage, administration time, administration method, etc. can be adjusted compared to Phase 2. The administration time is reduced to within 1 hour in the case of phase 3, and other methods such as inhalation as well as intravenous injection are being reviewed. In order to maintain the remaining amount of antibody in the body, it is judged that it is not meaningful to administer more than 40 mg of Rekirona vaccine.

In addition, Rekirona is effective against the basic Corona 19 virus and a variant virus originating in the UK, but the effect of a variant virus originating in South Africa is significantly reduced. Therefore, it is necessary to develop a new antibody. Accordingly, Celltrion is conducting research focusing on antibody No. 32 out of the 38 antibody candidate groups discovered.
Summarizing the explanation at the conference, in the case of a mutation from South Africa, it is a case of mutations from the amino acids at the ends of three spike proteins attached to the existing Corona 19 virus antigen. Therefore, it is estimated that the virus form has been modified compared to the British mutant virus, which mutated from the two spikes. Celltrion predicted that if an antibody to respond to mutations originating in South Africa, the remaining one or two spikes could be sufficiently effective against other viruses that have been modified. The clinical trial of an antibody candidate against mutations in South Africa is conducted alone in South Africa and takes about 6 months. Prior to that, animal clinical trials begin next month.
Honorary Chairman Seo said, “We are ready to make a vaccine (to fight mutation), and we can make any number of spike proteins.” Said.
Related Articles

Seo Jeong-jin Celltrion “can jump into the vaccine business for technology sovereignty”

Celltrion’Corona treatment’ started to supply free of charge to medical institutions

Celltrion licensed to sell’Humira biosimilar’ in Europe

Celltrion Pharmaceuticals, Remsima SC start sales in Korea
“The clinical trial for the South African mutation is only available in South Africa, and it has not yet spread in other countries.” “Cocktail therapy is intended to cover the South African mutation and use it as a multi-purpose to cover other mutations. I have to look it up.”
In addition, the honorary chairman Seo said, “(As I said in the beginning), the development cost of Rekkirona is expected to be 150 billion won, and the variation is also 150 billion won. He explained.