|
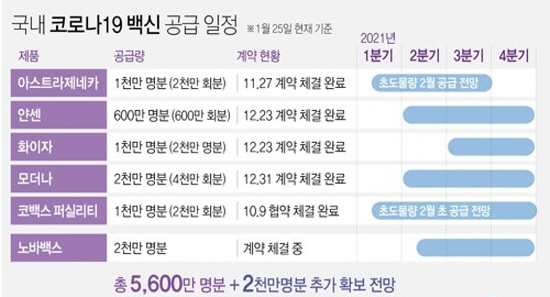
| ▲ According to the quarantine authorities on the 25th, the first quantity to come in among the 56 million people that the government has secured so far (76 million people including the planned contract amount) is’Covax Facility, an international project for joint purchase and distribution of vaccines. ‘This is the initial quantity of (COVAX facility). Subsequently, the AstraZeneca vaccine is expected to be introduced in the domestic market sequentially (photo source = Yonhap News). |
According to the pharmaceutical industry on the 30th, the Ministry of Food and Drug Safety held a’Corona 19 Vaccine Safety and Effectiveness Verification Advisory Group’ (hereinafter referred to as the Verification Advisory Group) meeting on the afternoon of the 31st with external experts regarding clinical trial data for AstraZeneca Corona 19 Vaccine (AZD1222). Open. The results will be released on February 1.
The Ministry of Food and Drug Safety has prepared a procedure for consulting external experts in’triple’ for objective and transparent approval review for COVID-19 treatments and vaccines. Consultation consists of the verification advisory group, the central pharmacist review committee (central pharmaceutical review), and the final inspection committee.
At the verification advisory meeting, clinical experts who focus on infectious medicine will participate to examine the safety and effectiveness of AstraZeneca Corona 19 vaccine, clinical significance, and the appropriateness of target patients.
AstraZeneca applied for an item permission for the Corona 19 vaccine with the Food and Drug Administration on January 4 this year. Among the corona 19 vaccines to be introduced in Korea, it is the first product to apply for product permission.
This product is a vaccine that is administered twice in total, and its storage conditions are 2-8℃, so it can be distributed under the existing system without the need to establish a separate cryogenic system.
It is expected that supply and demand will be smooth as it has signed a consignment production contract with SK Bioscience, a domestic vaccine company. Korea has signed a vaccine purchase contract with AstraZeneca for 10 million people.
However, it is known that the authorities will thoroughly verify this part as a controversy about the efficacy of the elderly over 65 has been raised.
The Ministry of Food and Drug Safety is comparing and reviewing the vaccination group and the placebo group to see if there is any safety information that requires special attention from the elderly over 65 years of age.
Copyright holder (c) Daily Good News. Unauthorized reproduction-redistribution prohibited