Announcement of the results of the 3rd therapeutic vaccine clinical support contest by the Ministry of Health and Welfare
Celid, selected for development of UBiologics vaccine
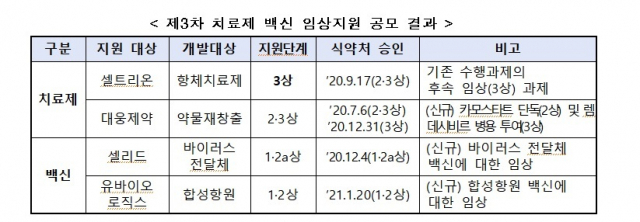
Celltrion and Daewoong Pharmaceutical support phase 2 and 3 treatments
 viewer
viewer
The government aims to develop a new coronavirus infection (Corona 19) treatment Celltrion (068270)·Daewoong Pharmaceutical (069620), Celid for vaccine developmentUbiologics (206650)Support.
The Ministry of Health and Welfare announced on the 26th that it would support the companies through the’National New Drug Development Project’.
Celltrion’s COVID-19 antibody treatment and Daewoong Pharmaceutical’s drug re-creation treatment will receive clinical support for phase 3 and phase 2 and 3, respectively. Celid and Ubiologics receive support for phase 1 and 2 clinical trials of vaccines.
The Ministry of Health and Welfare is conspiring bimonthly clinical support projects through the National New Drug Development Project Team to support the development of COVID-19 treatments and vaccines. In August and October last year, clinical support was provided for a total of 6 tasks, including 3 treatments and 3 vaccines, twice in total. Celltrion, Green Cross, and Daewoong Pharmaceutical were supported for the development of treatment, and Genexine, SK Bioscience, and Jinwon Life Science were supported for vaccine development. The government is not only working on budget but also supporting support to speed up development. Since September of last year, the’National Infectious Disease Clinical Trial Center’ has been operated to support recruitment of subjects. In addition, a consortium of hospitals dedicated to infectious diseases and base hospitals is formed to actively carry out clinical trials, and the’Clinical Trial Support TF’ composed of related ministries and private experts is operated to promptly improve corporate difficulties.
/ Reporter Ji-hye Seo [email protected]
< 저작권자 ⓒ 서울경제, 무단 전재 및 재배포 금지 >