 |
| © News1 Designer Choi Soo-ah |
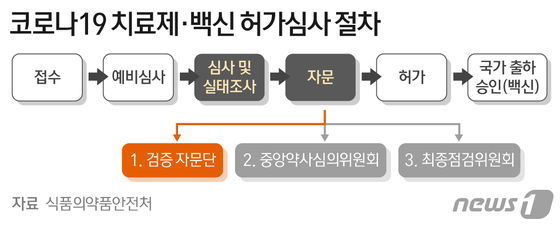
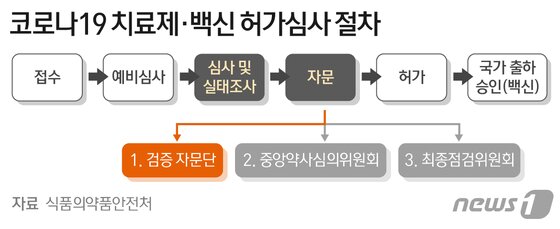
Celltrion’s’Corona 19′ antibody treatment,’Rekironaju (project name: CT-P59)’, received conditional approval recommendations from the verification advisory group of the Ministry of Food and Drug Safety, crossing the 9th ridge for final approval. As the result of the first official clinical evaluation, the Central Pharmacy Review Committee meeting and the final decision of the Ministry of Food and Drug Safety remain.
According to the Ministry of Food and Drug Safety on the 19th, the advisory group held a meeting on the 17th and confirmed the therapeutic effect and safety of the clinical phase 2 result of Rekkirona and recommended approval. Celltrion applied for conditional permission for the state of Rekirona on December 29 last year. This is a system that grants permission only with the results of phase 2 clinical trials under the condition that phase 3 clinical trials are separately implemented if there is no suitable treatment.
The recipients of the recommendation for prescription of Rekirona, as suggested by the advisory group, are those who are 18 years of age or older with mild to moderate’Corona 19′ confirmed. Under this condition, there is a high possibility that a final marketing approval will be issued.
Specifically, the advisory group was concerned with △People whose oxygen saturation exceeded 94% in indoor air △People who do not need supplementary oxygen supply △People who developed symptoms within 7 days prior to administration,’mild to moderate It recommended the efficacy and effect of improving clinical symptoms of’adult patients over 18 years of age’.
Oxygen saturation exceeding 94% means that there is no loss of lung function and oxygen therapy is not required. Taken together, patients who have no symptoms of Corona 19 or who are adults 18 years of age or older who are not severe and who have had symptoms for less than one week are eligible for treatment.
 |
| Kim Sang-bong, head of the Bio-Pharmaceutical Bureau of the Ministry of Food and Drug Safety, is announcing the results of the Celltrion novel coronavirus infection (Corona 19) treatment verification advisory meeting at the briefing room of the Centers for Disease Control and Prevention in Cheongju City, Chungbuk on the afternoon of the 18th. 2021.1.18/News1 © News1 Kim Yong-bin |
In particular, the advisory group confirmed that the period until recovery of Corona 19 symptoms in patients receiving Rekirona injection was 5.34 days, which was shortened by 3.43 days from 8.77 days in patients receiving placebo, and determined that there was a clinical effect. This is the result of evaluating how quickly the “recyronaju” group and the placebo group recover from 7 corona19 symptoms (fever, cough, difficulty breathing, sore throat, systemic pain (muscle pain), fatigue, and headache).
The reduction in the time between positive and negative transitions as a result of virus testing in the Rekirona injection group was not statistically significant. However, there was an opinion that a tendency to decrease the virus concentration in the body was observed after administration.
In addition, the ratio of patients who are hospitalized or need oxygen therapy due to Corona 19 has tended to decrease by administering Rekyrona in the’rate of inpatient hospitalization/oxygen therapy’, an auxiliary method for confirming the effect. The effect on the mortality rate has not been confirmed as none of the patients who received or did not die of Rekirona injection died. Regarding safety, mild or moderate adverse events occurred in general, but the ratio was similar when compared to the Rekirona injection group and the placebo group, and there were no significant life-threatening adverse events.
Therefore, it is expected that Rekironaju will help to smoothly operate and secure critical care beds by suppressing the transition to severe illness.
Joong-sik Eom, a professor of infectious medicine at Gachon University Gil Hospital, who participated in the clinical trial, announced the results of the second clinical trial for the first time at the 2021 High1 New Drug Development Symposium hosted by the Korean Pharmacy Association. This clinical trial proved that the rate of development into patients was significantly reduced while recovering at a rapid pace,” he said. “In order to prevent the spread of Corona 19 epidemic and the worsening of the situation, vaccines as well as treatments are essential options.”
However, the advisory group recommended additional phase 3 clinical trials of Rekirona to be confirmed more clearly. In particular, the incidence of inpatient/oxygen therapy patients decreased, but this is because, as it was an auxiliary confirmation device in this clinical trial, a separate statistical test method was not established.
The advisory group said, “In the future, it will be confirmed that a sufficient number of patients will be significantly reduced from mild to moderate to severe morbidity, and for patients who need supplemental oxygen therapy, Rekirona and existing severe treatments or other It was recommended to perform clinical trials in combination with immunomodulatory agents.
 |
| At the Celltrion 2 plant in Yeonsu-gu, Incheon, a researcher is looking at CT-P59, a corona19 antibody treatment. /News1 © News1 Reporter Jeong Jin-wook |