Celltrion·Healthcare·Pharmaceutical, shares fall 16~18% in two days
Corona 19 Antibody Treatment’Rekironaju’ Statistical Index Expectations Not Satisfying
“Reduction of critically ill patients and shortening of hospitalization period, etc., good for short time”

[서울파이낸스 남궁영진 기자] The sluggishness of the three Celltrion brothers is getting worse. This is due to disappointment in the clinical results of the recently announced new coronavirus infection (Corona 19) antibody treatment. Despite the degree of clinical performance, the statistical indicators did not meet expectations, and investor sentiment declined.
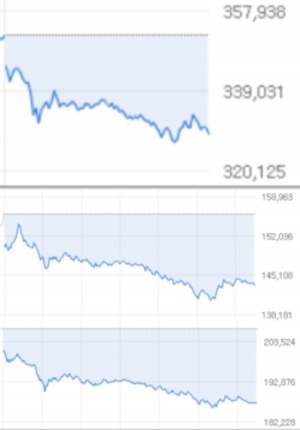
According to the Korea Exchange on the 15th, Celltrion ended with 329,000 won, down 23,500 won (6.67%) from the battlefield. It is the lowest level since November 24th last year (300,000 won). In the last two days, it fell to 14.27%, and the market capitalization ranking of the KOSPI market was also pushed from 7th to 9th.
Celltrion Healthcare, which has played a leading role in the KOSDAQ market, dropped 8.02% to 143,400 won, and Celltrion Pharmaceutical also dropped 9.51% to 18,7400 won. As a result, the three Celltrion brothers were named in the stock market that recorded the biggest drop on the day. The market cap evaporated over two days alone reaches 12,609.8 billion won.
After the deadline on the 13th, Celltrion announced the results of phase 2 clinical trials related to the Corona 19 antibody treatment’Rekirona’. Rekirona State said it reduced the incidence of severely ill patients requiring inpatient treatment by 54% in all patients and 68% in moderately ill patients aged 50 years and older, as well as shortening the recovery period by an average of 3 days or more.
However, it is observed that disappointing sales continued to pour out as the P value, a statistical index, showed a limit. P values of 0.05 or less are statistically significant. In the case of Rekirona, the subjects with moderate symptoms in their 50s had a P value of 0.0418. All patients (0.196%) and middle-aged pneumonia patients (0.105%) were less effective.
The analysis that it would not be of great help in improving performance also had an impact. Hana Financial Investment Researcher Seon Min-jung said, “There is a limit to expecting the effect of improving Celltrion’s performance due to Rekkirona.” Was diagnosed.
However, there are also positive reviews about this clinical trial. Lee Myung-sun, a researcher at Shinyoung Securities, said, “Celltrion’s Rekirona is superior in terms of safety compared to the antibody treatments of Eli Lilly and Regenenon, which were recently approved for emergency use by the US Food and Drug Administration (FDA). It is being reborn as a new drug development company.”
The researcher added, “This is a good performance considering the time to clinical trial itself, such as shortening the recovery rate of severe patients and reducing the number of visits to the hospital.” The event will drive the stock price,” he added.
Heo Hye-min, a researcher at Kiwoom Securities, said, “The second phase of Rekitona is particularly positive in that the hospitalization period has been shortened, especially in the elderly and high-risk groups,” he said. Suggested.
Celltrion applied for conditional approval from the Ministry of Food and Drug Safety on the 29th of last month based on the results of this phase 2 clinical trial, and is currently being reviewed by the Ministry of Food and Drug Safety.
An official from Celltrion said, “If Rekirona receives conditional permission from the Ministry of Food and Drug Safety, it has already completed production for 100,000 people and is thoroughly preparing a supply plan so that it can be supplied to the medical field immediately.” He said, “We plan to produce a cure for medicine.”

Copyright © Seoul Finance Unauthorized reproduction and redistribution prohibited