(Source = Hana Financial Investment)
On the 14th, Hana Financial Investment analyzed that there is a limit to expecting the effect of improving earnings for the new coronavirus infectious disease (Corona 19) treatment’Rekirona’ developed by Celltrion on the 14th.
The day before, Celltrion announced the results of global phase 2 clinical trials of Rekirona, a Corona 19 antibody treatment, at the ‘2021 High1 New Drug Development Symposium’. Based on the clinical results of a total of 307 patients, the recruited patients consisted of mild and severe outpatients, and 60% were moderately ill patients with pneumonia.
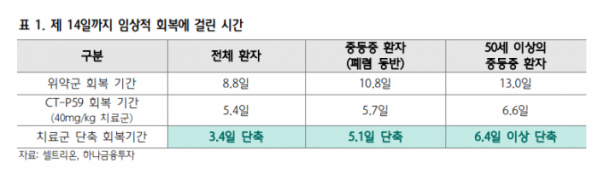
Clinical Results The incidence of developing mild and moderately severe patients requiring inpatient treatment decreased by 54% for all patients and 68% for moderately ill patients over 50 years of age compared to the placebo group in the receiving group (40mg/kg). The clinical recovery period was shortened by 3.4 days or more for all patients (8.8 days → 5.4 days), 5.1 days for moderately ill patients with pneumonia, and 6.4 days or more for moderately ill patients with pneumonia over 50 years old. Appeared.
Hana Financial Investment Research Institute Min-Jung Seon said, “There is a limit to expecting the effect of improving Celltrion’s performance with Rekkirona.” However, because the domestic market size is small, it will be difficult for the market to lead to an improvement in earnings.”
Researcher Sun said, “It is expected that it will enter the US and European markets in the future, but only 20% of the antibody treatment system of Regeneron, which has been effective in clinical results, is prescribed at local US hospitals.” The analysis that it is difficult to become a game changer is dominant, and it is time to make a more sober judgment on the treatment of Corona 19.”